Population physiologically-based pharmacokinetic model incorporating lymphatic uptake for a subcutaneously administered pegylated peptide
- PMID: 26932471
- PMCID: PMC4773320
- DOI: 10.1186/s40203-016-0018-5
Population physiologically-based pharmacokinetic model incorporating lymphatic uptake for a subcutaneously administered pegylated peptide
Abstract
Purpose: Physiologically-based pharmacokinetic (PBPK) models provide a rational mechanistic approach for predicting the time course of macromolecules in plasma. Population PBPK models for large molecules necessitate incorporation of lymphatic circulation to mechanistically account for biodistribution. Moreover, characterization of subcutaneous absorption requires consideration of the microvascular transit from the injection site to the systemic circulation. A PBPK model for a pegylated peptide conjugate, previously developed for primates, was modified to describe the lymphatic uptake in a population of humans by incorporation of interindividual variability in the lymphatic circulation and a unique lymphatic drainage compartment (LDC). The model was then used to simulate the time course of the drug in a population of humans and compared to the same drug administered to a group of human subjects participating in a first-in-human study.
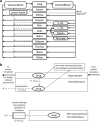
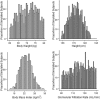
Methods: Organ, blood and lymph masses for the population were sampled from either normal or log-normal distributions. Blood flows were calculated for each organ based on mean organ perfusion per gram of organ tissue and lymphatic flow was set as a fixed fraction of blood flow. Interindividual variability in lymphatic volume was assumed to be similar to that of blood volume. The volume of the LDC was parameterzed as a fraction of the injection volume. Sensitivity analysis was performed to study uncertain parameters and distribution assumptions.
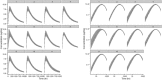
Results: The population generator was capable of simulating a virtual population incorporating the lymphatic circulation. Incorporation of a LDC resulted in similar line shape relative to the observed data and incorporation of anthropometric variability accounted for individual differences in the absorption and elimination phases across all dose cohorts. Line shape was sensitive to the inclusion of LDC while peak and elimination portions of the time course were influenced by the magnitude of variance assumed for blood volume and renal clearance, respectively.
Conclusion: Lymphatic circulation can be incorporated into a population PBPK model assuming similar interindividual variability as observed for blood volume. Incorporation of an LDC, where the volume of this transit compartment is proportional to the SC injection volume may be an important mechanistic means of predicting the transit from the SC depot to the systemic circulation.
Keywords: Lymph; Pegylated; Peptide; Physiologically-based pharmacokinetic model; Subcutaneous.
Figures


Similar articles
-
A PBPK workflow for first-in-human dose selection of a subcutaneously administered pegylated peptide.J Pharmacokinet Pharmacodyn. 2015 Apr;42(2):135-50. doi: 10.1007/s10928-015-9406-4. Epub 2015 Feb 4. J Pharmacokinet Pharmacodyn. 2015. PMID: 25650156
-
Understanding the Monoclonal Antibody Disposition after Subcutaneous Administration using a Minimal Physiologically based Pharmacokinetic Model.J Pharm Pharm Sci. 2018;21(1s):130s-148s. doi: 10.18433/jpps30028. J Pharm Pharm Sci. 2018. PMID: 30011390 Free PMC article.
-
A physiologically based pharmacokinetic drug-disease model to predict carvedilol exposure in adult and paediatric heart failure patients by incorporating pathophysiological changes in hepatic and renal blood flows.Clin Pharmacokinet. 2015 Sep;54(9):943-62. doi: 10.1007/s40262-015-0253-7. Clin Pharmacokinet. 2015. PMID: 25773479 Free PMC article.
-
Physiologically Based Pharmacokinetic Modeling of Therapeutic Proteins.J Pharm Sci. 2017 Sep;106(9):2270-2275. doi: 10.1016/j.xphs.2017.03.038. Epub 2017 Apr 7. J Pharm Sci. 2017. PMID: 28392453 Review.
-
Database for physiologically based pharmacokinetic (PBPK) modeling: physiological data for healthy and health-impaired elderly.J Toxicol Environ Health B Crit Rev. 2009 Jan;12(1):1-24. doi: 10.1080/10937400802545060. J Toxicol Environ Health B Crit Rev. 2009. PMID: 19117207 Review.
Cited by
-
A PBPK model describing the pharmacokinetics of γ-HBCD exposure in mice.Toxicol Appl Pharmacol. 2021 Oct 1;428:115678. doi: 10.1016/j.taap.2021.115678. Epub 2021 Aug 11. Toxicol Appl Pharmacol. 2021. PMID: 34390738 Free PMC article.
-
Predicting monoclonal antibody pharmacokinetics following subcutaneous administration via whole-body physiologically-based modeling.J Pharmacokinet Pharmacodyn. 2020 Oct;47(5):385-409. doi: 10.1007/s10928-020-09691-3. Epub 2020 Jun 4. J Pharmacokinet Pharmacodyn. 2020. PMID: 32500362 Free PMC article.
-
A modeling platform for the lymphatic system.J Theor Biol. 2020 May 21;493:110193. doi: 10.1016/j.jtbi.2020.110193. Epub 2020 Feb 28. J Theor Biol. 2020. PMID: 32119968 Free PMC article.
-
Mathematical Models to Characterize the Absorption, Distribution, Metabolism, and Excretion of Protein Therapeutics.Drug Metab Dispos. 2022 Jun;50(6):867-878. doi: 10.1124/dmd.121.000460. Epub 2022 Feb 23. Drug Metab Dispos. 2022. PMID: 35197311 Free PMC article. Review.
-
A new paradigm for drug discovery in the treatment of complex diseases: drug discovery and optimization.Chin Med. 2025 Mar 24;20(1):40. doi: 10.1186/s13020-025-01075-4. Chin Med. 2025. PMID: 40122800 Free PMC article. Review.
References
-
- Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54:1517–1528. - PubMed
-
- Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55:4611–4622. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

