Myocardial pathology induced by aldosterone is dependent on non-canonical activities of G protein-coupled receptor kinases
- PMID: 26932512
- PMCID: PMC4778065
- DOI: 10.1038/ncomms10877
Myocardial pathology induced by aldosterone is dependent on non-canonical activities of G protein-coupled receptor kinases
Abstract
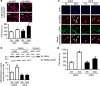
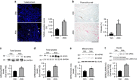
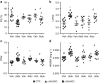
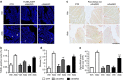
Hyper-aldosteronism is associated with myocardial dysfunction including induction of cardiac fibrosis and maladaptive hypertrophy. Mechanisms of these cardiotoxicities are not fully understood. Here we show that mineralocorticoid receptor (MR) activation by aldosterone leads to pathological myocardial signalling mediated by mitochondrial G protein-coupled receptor kinase 2 (GRK2) pro-death activity and GRK5 pro-hypertrophic action. Moreover, these MR-dependent GRK2 and GRK5 non-canonical activities appear to involve cross-talk with the angiotensin II type-1 receptor (AT1R). Most importantly, we show that ventricular dysfunction caused by chronic hyper-aldosteronism in vivo is completely prevented in cardiac Grk2 knockout mice (KO) and to a lesser extent in Grk5 KO mice. However, aldosterone-induced cardiac hypertrophy is totally prevented in Grk5 KO mice. We also show human data consistent with MR activation status in heart failure influencing GRK2 levels. Therefore, our study uncovers GRKs as targets for ameliorating pathological cardiac effects associated with high-aldosterone levels.
Figures
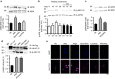
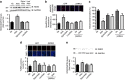
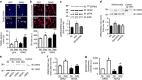
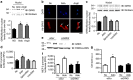

References
-
- Briet M. & Schiffrin E. L. Aldosterone: effects on the kidney and cardiovascular system. Nat. Rev. Nephrol. 6, 261–273 (2010). - PubMed
-
- Swedberg K., Eneroth P., Kjekshus J. & Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation 82, 1730–1736 (1990). - PubMed
-
- Jorde U. P. et al.. Elevated plasma aldosterone levels despite complete inhibition of the vascular angiotensin-converting enzyme in chronic heart failure. Circulation 106, 1055–1057 (2002). - PubMed
-
- Gutierrez-Marcos F. M. et al.. Atrial natriuretic peptide in patients with acute myocardial infarction without functional heart failure. Eur. Heart J. 12, 503–507 (1991). - PubMed
-
- Yoshida M. et al.. Mineralocorticoid receptor is overexpressed in cardiomyocytes of patients with congestive heart failure. Congest. Heart Fail. 11, 12–16 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

