Strategies and Challenges to Myocardial Replacement Therapy
- PMID: 26933042
- PMCID: PMC4798743
- DOI: 10.5966/sctm.2015-0288
Strategies and Challenges to Myocardial Replacement Therapy
Abstract
Cardiovascular diseases account for the majority of deaths globally and are a significant drain on economic resources. Although heart transplants and left-ventricle assist devices are the solution for some, the best chance for many patients who suffer because of a myocardial infarction, heart failure, or a congenital heart disease may be cell-based regenerative therapies. Such therapies can be divided into two categories: the application of a cell suspension and the implantation of an in vitro engineered tissue construct to the damaged area of the heart. Both strategies have their advantages and challenges, and in this review, we discuss the current state of the art in myocardial regeneration, the challenges to success, and the future direction of the field.
Significance: This article outlines the advantages and limitations of the cell injection and patch approaches to cardiac regenerative therapy. If the field is to move forward, some fundamental questions require answers, including the limitations to the use of animal models for human cell-transplantation studies; the best way to measure success in terms of functional improvements, histological integration, electrical coupling, and arrhythmias; and where the cells should be applied for maximal benefit-the epicardium or the myocardium.
©AlphaMed Press.
Figures
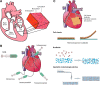
References
-
- Mendis S, Davis S, Norrving B. Organizational update: The World Health Organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46:e121–e122. - PubMed
-
- Tous E, Purcell B, Ifkovits JL, et al. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res. 2011;4:528–542. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

