Cytokinesis involves a nontranscriptional function of the Hippo pathway effector YAP
- PMID: 26933062
- PMCID: PMC5455055
- DOI: 10.1126/scisignal.aaa9227
Cytokinesis involves a nontranscriptional function of the Hippo pathway effector YAP
Abstract
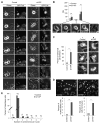
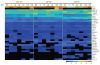
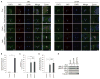
YAP is a transcriptional coactivator that controls organ expansion and differentiation and is inhibited by the Hippo pathway in cells in interphase. Here, we demonstrated that, during mitosis, YAP localized to the midbody and spindle, subcellular structures that are involved in cytokinesis, the process by which contraction of the cytoskeleton produces two daughter cells. Furthermore, YAP was phosphorylated by CDK1, a kinase that promotes cell cycle progression. Knockdown of YAP by shRNA or expression of a nonphosphorylatable form of YAP delayed the separation of daughter cells (called abscission) and induced a cytokinesis phenotype associated with increased contractile force, membrane blebbing and bulges, and abnormal spindle orientation. Consequently, these defects led to an increased frequency of multinucleation, micronuclei, and aneuploidy. YAP was required for proper localization of proteins that regulate contraction during cytokinesis, including ECT2, MgcRacGap, Anillin, and RHOA. In addition, depletion of YAP increased the phosphorylation of myosin light chain, which would be expected to activate the contractile activity of myosin II, the molecular motor involved in cytokinesis. The polarity scaffold protein PATJ coprecipitated with YAP and colocalized with YAP at the cytokinesis midbody, and knockdown of PATJ phenocopied the cytokinetic defects and spindle orientation alterations induced by either YAP depletion or expression of a nonphosphorylatable YAP mutant. Together, these results reveal an unanticipated role for YAP in the proper organization of the cytokinesis machinery during mitosis through interaction with the polarity protein PATJ.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures




Comment in
-
Properly dividing with YAP.Sci Signal. 2016 Mar 1;9(417):fs3. doi: 10.1126/scisignal.aaf2527. Sci Signal. 2016. PMID: 26933061
Similar articles
-
Overexpression of Rac GTPase Activating Protein 1 Contributes to Proliferation of Cancer Cells by Reducing Hippo Signaling to Promote Cytokinesis.Gastroenterology. 2018 Oct;155(4):1233-1249.e22. doi: 10.1053/j.gastro.2018.07.010. Epub 2018 Sep 5. Gastroenterology. 2018. PMID: 30009820
-
MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis.Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13158-63. doi: 10.1073/pnas.0504145102. Epub 2005 Aug 29. Proc Natl Acad Sci U S A. 2005. PMID: 16129829 Free PMC article.
-
Inhibition of cyclin-dependent kinase 1 induces cytokinesis without chromosome segregation in an ECT2 and MgcRacGAP-dependent manner.J Biol Chem. 2005 Oct 28;280(43):36502-9. doi: 10.1074/jbc.M508007200. Epub 2005 Aug 23. J Biol Chem. 2005. PMID: 16118207
-
Hippo-yap signaling in ocular development and disease.Dev Dyn. 2018 Jun;247(6):794-806. doi: 10.1002/dvdy.24628. Epub 2018 Apr 23. Dev Dyn. 2018. PMID: 29532607 Free PMC article. Review.
-
Anillin: a pivotal organizer of the cytokinetic machinery.Biochem Soc Trans. 2008 Jun;36(Pt 3):439-41. doi: 10.1042/BST0360439. Biochem Soc Trans. 2008. PMID: 18481976 Free PMC article. Review.
Cited by
-
Roles of Membrane and Vesicular Traffic in Regulation of the Hippo Pathway.Front Cell Dev Biol. 2020 Jan 10;7:384. doi: 10.3389/fcell.2019.00384. eCollection 2019. Front Cell Dev Biol. 2020. PMID: 32010696 Free PMC article. Review.
-
The CRB1 Complex: Following the Trail of Crumbs to a Feasible Gene Therapy Strategy.Front Neurosci. 2017 Apr 5;11:175. doi: 10.3389/fnins.2017.00175. eCollection 2017. Front Neurosci. 2017. PMID: 28424578 Free PMC article. Review.
-
TPX2 overexpression promotes sensitivity to dasatinib in breast cancer by activating YAP transcriptional signaling.Mol Oncol. 2024 Jun;18(6):1531-1551. doi: 10.1002/1878-0261.13602. Epub 2024 Feb 15. Mol Oncol. 2024. PMID: 38357786 Free PMC article.
-
Inhibition of cyclin-dependent kinase 7 down-regulates yes-associated protein expression in mesothelioma cells.J Cell Mol Med. 2020 Jan;24(1):1087-1098. doi: 10.1111/jcmm.14841. Epub 2019 Nov 21. J Cell Mol Med. 2020. PMID: 31755214 Free PMC article.
-
Overexpression of epithelial cell transforming 2 protein in colorectal carcinoma predicts a poor prognosis.Exp Ther Med. 2017 Nov;14(5):4862-4868. doi: 10.3892/etm.2017.5132. Epub 2017 Sep 19. Exp Ther Med. 2017. PMID: 29109759 Free PMC article.
References
-
- Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–2152. - PubMed
-
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

