Architectural transitions in Vibrio cholerae biofilms at single-cell resolution
- PMID: 26933214
- PMCID: PMC4833255
- DOI: 10.1073/pnas.1601702113
Architectural transitions in Vibrio cholerae biofilms at single-cell resolution
Abstract
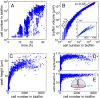
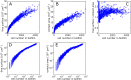
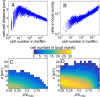
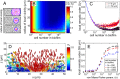
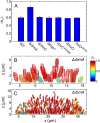
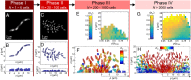
Many bacterial species colonize surfaces and form dense 3D structures, known as biofilms, which are highly tolerant to antibiotics and constitute one of the major forms of bacterial biomass on Earth. Bacterial biofilms display remarkable changes during their development from initial attachment to maturity, yet the cellular architecture that gives rise to collective biofilm morphology during growth is largely unknown. Here, we use high-resolution optical microscopy to image all individual cells in Vibrio cholerae biofilms at different stages of development, including colonies that range in size from 2 to 4,500 cells. From these data, we extracted the precise 3D cellular arrangements, cell shapes, sizes, and global morphological features during biofilm growth on submerged glass substrates under flow. We discovered several critical transitions of the internal and external biofilm architectures that separate the major phases of V. cholerae biofilm growth. Optical imaging of biofilms with single-cell resolution provides a new window into biofilm formation that will prove invaluable to understanding the mechanics underlying biofilm development.
Keywords: biofilm; community; emergent order; nematic order; self-organization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Comment in
-
Three-dimensional architecture of Vibrio cholera biofilms.Proc Natl Acad Sci U S A. 2016 Apr 5;113(14):3711-3. doi: 10.1073/pnas.1603016113. Epub 2016 Mar 24. Proc Natl Acad Sci U S A. 2016. PMID: 27036000 Free PMC article. No abstract available.
References
-
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. - PubMed
-
- Parsek MR, Greenberg EP. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005;13(1):27–33. - PubMed
-
- Römling U, et al. Microbial biofilm formation: A need to act. J Intern Med. 2014;276(2):98–110. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

