A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application
- PMID: 26933740
- PMCID: PMC4779185
- DOI: 10.1016/j.chembiol.2015.12.003
A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application
Abstract
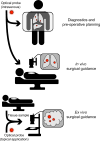
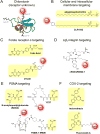
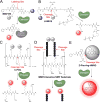
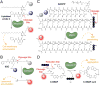
The Precision Medicine Initiative aims to use advances in basic and clinical research to develop therapeutics that selectively target and kill cancer cells. Under the same doctrine of precision medicine, there is an equally important need to visualize these diseased cells to enable diagnosis, facilitate surgical resection, and monitor therapeutic response. Therefore, there is a great opportunity for chemists to develop chemically tractable probes that can image cancer in vivo. This review focuses on recent advances in the development of optical probes, as well as their current and future applications in the clinical management of cancer. The progress in probe development described here suggests that optical imaging is an important and rapidly developing field of study that encourages continued collaboration among chemists, biologists, and clinicians to further refine these tools for interventional surgical imaging, as well as for diagnostic and therapeutic applications.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
-
- Andreesen R, Modolell M, Weltzien HU, Eibl H, Common HH, Lohr GW, Munder PG. Selective destruction of human leukemic cells by alkyl-lysophospholipids. Cancer Res. 1978;38:3894–3899. - PubMed
-
- Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, et al. A small-molecule dye for NIR-II imaging. Nat Mater 2015 - PubMed
-
- Antony AC. Folate receptors. Annu Rev Nutr. 1996;16:501–521. - PubMed
-
- Aoki T, Murakami M, Yasuda D, Shimizu Y, Kusano T, Matsuda K, Niiya T, Kato H, Murai N, Otsuka K, et al. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Sci. 2010;17:590–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

