Neutrophil migration into the placenta: Good, bad or deadly?
- PMID: 26933824
- PMCID: PMC4853040
- DOI: 10.1080/19336918.2016.1148866
Neutrophil migration into the placenta: Good, bad or deadly?
Abstract
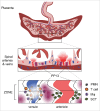
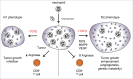
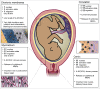
Almost 2 decades have passed since the discovery that pregnancy is associated with a basal inflammatory state involving neutrophil activation, and that this is more overt in cases with preeclampsia, than in instances with sepsis. This pivotal observation paved the way for our report, made almost a decade ago, describing the first involvement of neutrophil extracellular traps (NETs) in a non-infectious human pathology, namely preeclampsia, where an abundance of these structures were detected directly in the placental intervillous space. Despite these remarkable findings, there remains a paucity of interest among reproductive biologists in further exploring the role or involvement of neutrophils in pregnancy and related pathologies. In this review we attempt to redress this deficit by highlighting novel recent findings including the discovery of a novel neutrophil subset in the decidua, the interaction of placental protein 13 (PP13) and neutrophils in modulating spiral artery modification, as well as the use of animal model systems to elucidate neutrophil function in implantation, gestation and parturition. These model systems have been particularly useful in identifying key components implicated in recurrent fetal loss, preeclampsia or new signaling molecules such as sphingolipids. Finally, the recent discovery that anti-phospolipid antibodies can trigger NETosis, supports our hypothesis that these structures may contribute to placental dysfunction in pertinent cases with recurrent fetal loss.
Keywords: animal model; neutrophil extracellular traps (NETs); parturition; preeclampsia; pregnancy; recurrent fetal loss.
Figures


References
-
- Borregaard N. Neutrophils, from marrow to microbes. Immunity 2010; 33:657-70; PMID:21094463; http://dx.doi.org/10.1016/j.immuni.2010.11.011 - DOI - PubMed
-
- Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 2013; 210:1283-99; PMID:23825232; http://dx.doi.org/10.1084/jem.20122220 - DOI - PMC - PubMed
-
- Hahn S, Giaglis S, Chowdhury CS, Hosli I, Hasler P. Modulation of neutrophil NETosis: interplay between infectious agents and underlying host physiology. Semin Immunopathol 2013; 35:439-53; PMID:23649713; http://dx.doi.org/10.1007/s00281-013-0380-x - DOI - PMC - PubMed
-
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, et al.. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007; 449:564-9; PMID:17873860; http://dx.doi.org/10.1038/nature06116 - DOI - PubMed
-
- Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, et al.. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011; 3:73ra19; PMID:21389263; http://dx.doi.org/10.1126/scitranslmed.3001180 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
