Reproduction of the FC/DFC units in nucleoli
- PMID: 26934002
- PMCID: PMC4916892
- DOI: 10.1080/19491034.2016.1157674
Reproduction of the FC/DFC units in nucleoli
Abstract
The essential structural components of the nucleoli, Fibrillar Centers (FC) and Dense Fibrillar Components (DFC), together compose FC/DFC units, loci of rDNA transcription and early RNA processing. In the present study we followed cell cycle related changes of these units in 2 human sarcoma derived cell lines with stable expression of RFP-PCNA (the sliding clamp protein) and GFP-RPA43 (a subunit of RNA polymerase I, pol I) or GFP-fibrillarin. Correlative light and electron microscopy analysis showed that the pol I and fibrillarin positive nucleolar beads correspond to individual FC/DFC units. In vivo observations showed that at early S phase, when transcriptionally active ribosomal genes were replicated, the number of the units in each cell increased by 60-80%. During that period the units transiently lost pol I, but not fibrillarin. Then, until the end of interphase, number of the units did not change, and their duplication was completed only after the cell division, by mid G1 phase. This peculiar mode of reproduction suggests that a considerable subset of ribosomal genes remain transcriptionally silent from mid S phase to mitosis, but become again active in the postmitotic daughter cells.
Keywords: FC/DFC units; cell cycle; nucleolus; rDNA; replication.
Figures
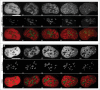



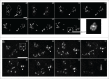


References
-
- Henderson AS, Warburton D, Atwood KC. Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci USA 1972; 69:3394-8; PMID:4508329; http://dx.doi.org/10.1073/pnas.69.11.3394 - DOI - PMC - PubMed
-
- Long EO, Dawid IB. Repeated genes in eukaryotes. Annu Rev Biochem 1980; 49:727-64; PMID:6996571; http://dx.doi.org/10.1146/annurev.bi.49.070180.003455 - DOI - PubMed
-
- Puvion-Dutilleul F, Bachellerie JP, Puvion E. Nucleolar organization of HeLa cells as studied by in situ hybridization. Chromosoma 1991; 100:395-409; PMID:1893795; http://dx.doi.org/10.1007/BF00337518 - DOI - PubMed
-
- Raska I. Oldies but goldies: searching for Christmas trees within the nucleolar architecture. Trends Cell Biol 2003; 13:517-25; PMID:14507479; http://dx.doi.org/10.1016/j.tcb.2003.08.003 - DOI - PubMed
-
- Raska I, Shaw PJ, Cmarko D. New insights into nucleolar architecture and activity. Int Rev Cytol 2006a; 255:177-235; http://dx.doi.org/10.1016/S0074-7696(06)55004-1 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
