NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis
- PMID: 26934227
- PMCID: PMC4786464
- DOI: 10.1038/nature16969
NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis
Abstract
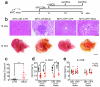
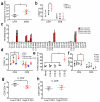
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death. Non-alcoholic fatty liver disease (NAFLD) affects a large proportion of the US population and is considered to be a metabolic predisposition to liver cancer. However, the role of adaptive immune responses in NAFLD-promoted HCC is largely unknown. Here we show, in mouse models and human samples, that dysregulation of lipid metabolism in NAFLD causes a selective loss of intrahepatic CD4(+) but not CD8(+) T lymphocytes, leading to accelerated hepatocarcinogenesis. We also demonstrate that CD4(+) T lymphocytes have greater mitochondrial mass than CD8(+) T lymphocytes and generate higher levels of mitochondrially derived reactive oxygen species (ROS). Disruption of mitochondrial function by linoleic acid, a fatty acid accumulated in NAFLD, causes more oxidative damage than other free fatty acids such as palmitic acid, and mediates selective loss of intrahepatic CD4(+) T lymphocytes. In vivo blockade of ROS reversed NAFLD-induced hepatic CD4(+) T lymphocyte decrease and delayed NAFLD-promoted HCC. Our results provide an unexpected link between lipid dysregulation and impaired anti-tumour surveillance.
Figures
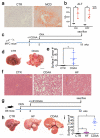



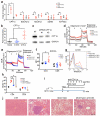
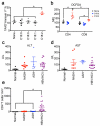



Comment in
-
CD4+ T Cell Loss Drives NAFLD Progression to Hepatocellular Carcinoma.Cancer Discov. 2016 May;6(5):OF8. doi: 10.1158/2159-8290.CD-RW2016-046. Epub 2016 Mar 17. Cancer Discov. 2016. PMID: 26988666
-
NAFLD: Loss of CD4(+) T cells in HCC.Nat Rev Gastroenterol Hepatol. 2016 Apr;13(4):190. doi: 10.1038/nrgastro.2016.47. Epub 2016 Mar 16. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27006250 No abstract available.
-
Fatty Acid-Induced T Cell Loss Greases Liver Carcinogenesis.Cell Metab. 2016 May 10;23(5):759-61. doi: 10.1016/j.cmet.2016.04.018. Cell Metab. 2016. PMID: 27166937
-
Hepatocellular carcinoma in nonalcoholic fatty liver disease: A link between oxidative stress and T-cell suppression.Hepatology. 2016 Nov;64(5):1794-1797. doi: 10.1002/hep.28815. Hepatology. 2016. PMID: 27629157 No abstract available.
-
Immune surveillance of liver cancer in non-alcoholic fatty liver disease: excess lipids cause CD4 T-cells loss and promote hepatocellular carcinoma development.Hepatobiliary Surg Nutr. 2016 Oct;5(5):433-437. doi: 10.21037/hbsn.2016.09.10. Hepatobiliary Surg Nutr. 2016. PMID: 27826559 Free PMC article. No abstract available.
-
Inflammation, mitochondrial metabolism and nutrition: the multi-faceted progression of non-alcoholic fatty liver disease to hepatocellular carcinoma.Hepatobiliary Surg Nutr. 2016 Oct;5(5):438-443. doi: 10.21037/hbsn.2016.09.11. Hepatobiliary Surg Nutr. 2016. PMID: 27826560 Free PMC article. No abstract available.
-
Comments on "NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis".Hepatobiliary Surg Nutr. 2016 Dec;5(6):509-510. doi: 10.21037/hbsn.2016.12.02. Hepatobiliary Surg Nutr. 2016. PMID: 28124010 Free PMC article. No abstract available.
-
New players in non-alcoholic fatty liver disease induced carcinogenesis: lipid dysregulation impairs liver immune surveillance.Hepatobiliary Surg Nutr. 2016 Dec;5(6):511-514. doi: 10.21037/hbsn.2016.11.08. Hepatobiliary Surg Nutr. 2016. PMID: 28124011 Free PMC article. No abstract available.
Similar articles
-
Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4+ T cell apoptosis promoting HCC development.Cell Death Dis. 2018 May 23;9(6):620. doi: 10.1038/s41419-018-0687-6. Cell Death Dis. 2018. PMID: 29795111 Free PMC article.
-
Nonalcoholic fatty liver disease promotes hepatocellular carcinoma through direct and indirect effects on hepatocytes.FEBS J. 2018 Feb;285(4):752-762. doi: 10.1111/febs.14209. Epub 2017 Sep 15. FEBS J. 2018. PMID: 28857485 Review.
-
[Non alcoholic-fatty liver disease causes selective CD4+ T lymphocytes loss and promotes hepatocarcinogenesis].Med Sci (Paris). 2016 Nov;32(11):1023-1026. doi: 10.1051/medsci/20163211021. Epub 2016 Dec 23. Med Sci (Paris). 2016. PMID: 28008846 French. No abstract available.
-
Peroxisomal β-oxidation regulates whole body metabolism, inflammatory vigor, and pathogenesis of nonalcoholic fatty liver disease.JCI Insight. 2018 Mar 22;3(6):e93626. doi: 10.1172/jci.insight.93626. JCI Insight. 2018. PMID: 29563328 Free PMC article.
-
Deranged hepatocyte intracellular Ca2+ homeostasis and the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma.Cell Calcium. 2019 Sep;82:102057. doi: 10.1016/j.ceca.2019.102057. Epub 2019 Jul 26. Cell Calcium. 2019. PMID: 31401389 Review.
Cited by
-
Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis.ESMO Open. 2022 Dec;7(6):100591. doi: 10.1016/j.esmoop.2022.100591. Epub 2022 Oct 6. ESMO Open. 2022. PMID: 36208496 Free PMC article.
-
Comprehensive analysis of SLC43A2 on the tumor immune microenvironment and prognosis of liver hepatocellular carcinoma.Front Genet. 2022 Sep 16;13:911378. doi: 10.3389/fgene.2022.911378. eCollection 2022. Front Genet. 2022. PMID: 36186480 Free PMC article.
-
CD5L-associated gene analyses highlight the dysregulations, prognostic effects, immune associations, and drug-sensitivity predicative potentials of LCAT and CDC20 in hepatocellular carcinoma.Cancer Cell Int. 2022 Dec 9;22(1):393. doi: 10.1186/s12935-022-02820-7. Cancer Cell Int. 2022. PMID: 36494696 Free PMC article.
-
Integrative transcriptome analysis identifies a crotonylation gene signature for predicting prognosis and drug sensitivity in hepatocellular carcinoma.J Cell Mol Med. 2024 Oct;28(20):e70083. doi: 10.1111/jcmm.70083. J Cell Mol Med. 2024. PMID: 39428564 Free PMC article.
-
Evaluation of the prognostic value of paraoxonase 1 in the recurrence and metastasis of hepatocellular carcinoma and establishment of a liver-specific predictive model of survival.J Transl Med. 2018 Nov 26;16(1):327. doi: 10.1186/s12967-018-1707-0. J Transl Med. 2018. PMID: 30477582 Free PMC article.
References
Additonal References for Methods
-
- Radaeva S, et al. Interferon-alpha activates multiple STAT signals and down-regulates c-Met in primary human hepatocytes. Gastroenterology. 2002;122:1020–1034. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

