Clinical evaluation, biochemistry and genetic polymorphism analysis for the diagnosis of lactose intolerance in a population from northeastern Brazil
- PMID: 26934237
- PMCID: PMC4763107
- DOI: 10.6061/clinics/2016(02)06
Clinical evaluation, biochemistry and genetic polymorphism analysis for the diagnosis of lactose intolerance in a population from northeastern Brazil
Abstract
Objective: This work aimed to evaluate and correlate symptoms, biochemical blood test results and single nucleotide polymorphisms for lactose intolerance diagnosis.
Method: A cross-sectional study was conducted in Fortaleza, Ceará, Brazil, with a total of 119 patients, 54 of whom were lactose intolerant. Clinical evaluation and biochemical blood tests were conducted after lactose ingestion and blood samples were collected for genotyping evaluation. In particular, the single nucleotide polymorphisms C>T-13910 and G>A-22018 were analyzed by restriction fragment length polymorphism/polymerase chain reaction and validated by DNA sequencing.
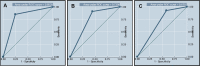
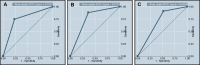
Results: Lactose-intolerant patients presented with more symptoms of flatulence (81.4%), bloating (68.5%), borborygmus (59.3%) and diarrhea (46.3%) compared with non-lactose-intolerant patients (p<0.05). We observed a significant association between the presence of the alleles T-13910 and A-22018 and the lactose-tolerant phenotype (p<0.05). After evaluation of the biochemical blood test results for lactose, we found that the most effective cutoff for glucose levels obtained for lactose malabsorbers was <15 mg/dL, presenting an area under the receiver operating characteristic curve greater than 80.3%, with satisfactory values for sensitivity and specificity.
Conclusions: These data corroborate the association of these single nucleotide polymorphisms (C>T-13910 and G>A-22018) with lactose tolerance in this population and suggest clinical management for patients with lactose intolerance that considers single nucleotide polymorphism detection and a change in the biochemical blood test cutoff from <25 mg/dL to <15 mg/dL.
Conflict of interest statement
No potential conflict of interest was reported.
Figures
References
-
- Levitt M, Wilt T, Shaukat A. Clinical implications of lactose malabsorption versus lactose intolerance. J Clin Gastroenterol. 2013;47((6)):471–80. 10.1097/MCG.0b013e3182889f0f - DOI - PubMed
-
- Rienzo TD, D'Angelo G, D'Aversa F, Campanale MC, Cesario V, Montalto M, et al. Lactose Intolerance: from diagnosis to correct management. Eur Rev Med Pharmacol Sci. 2013;17((Suppl 2)):18–25. - PubMed
-
- Mattar R, Mazo DFC, Carrilho FJ. Lactose intolerance: diagnosis, genetic and clinical factors. Clin Exp Gastroenterol. 2012;5:113–21. 10.2147/CEG.S32368 - DOI - PMC - PubMed
-
- Lomer MCE, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice – myths and realities. Aliment Pharmacol Ther. 2008;27((2)):93–103. 10.1111/j.1365-2036.2007.03557.x - DOI - PubMed
-
- Misselwitz B. Lactose Intolerance: New Insights due to Blinded Testing? Digestion. 2014;90((1)):72–3. 10.1159/000365144 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

