ER maleate is a novel anticancer agent in oral cancer: implications for cancer therapy
- PMID: 26934445
- PMCID: PMC4941378
- DOI: 10.18632/oncotarget.7751
ER maleate is a novel anticancer agent in oral cancer: implications for cancer therapy
Abstract
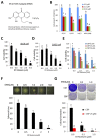
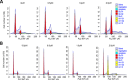
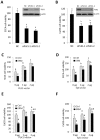
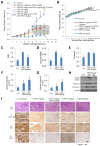
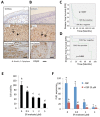
ER maleate [10-(3-Aminopropyl)-3, 4-dimethyl-9(10H)-acridinone maleate] identified in a kinome screen was investigated as a novel anticancer agent for oral squamous cell carcinoma (OSCC). Our aim was to demonstrate its anticancer effects, identify putative molecular targets and determine their clinical relevance and investigate its chemosensitization potential for platinum drugs to aid in OSCC management. Biologic effects of ER maleate were determined using oral cancer cell lines in vitro and oral tumor xenografts in vivo. mRNA profiling, real time PCR and western blot revealed ER maleate modulated the expression of polo-like kinase 1 (PLK1) and spleen tyrosine kinase (Syk). Their clinical significance was determined in oral SCC patients by immunohistochemistry and correlated with prognosis by Kaplan-Meier survival and multivariate Cox regression analyses. ER maleate induced cell apoptosis, inhibited proliferation, colony formation, migration and invasion in oral cancer cells. Imagestream analysis revealed cell cycle arrest in G2/M phase and increased polyploidy, unravelling deregulation of cell division and cell death. Mechanistically, ER maleate decreased expression of PLK1 and Syk, induced cleavage of PARP, caspase9 and caspase3, and increased chemosensitivity to carboplatin; significantly suppressed tumor growth and increased antitumor activity of carboplatin in tumor xenografts. ER maleate treated tumor xenografts showed reduced PLK1 and Syk expression. Clinical investigations revealed overexpression of PLK1 and Syk in oral SCC patients that correlated with disease prognosis. Our in vitro and in vivo findings provide a strong rationale for pre-clinical efficacy of ER maleate as a novel anticancer agent and chemosensitizer of platinum drugs for OSCC.
Keywords: ER maleate; OSCC; Syk / PLK1; anticancer agent; tumor xenografts.
Conflict of interest statement
RR and PGW are shareholders in Proteocyte Diagnostics Inc., all the other coauthors declared no potential conflicts of interest.
Figures





References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. - PubMed
-
- Scully C, Bagan JV. Recent advances in Oral Oncology 2007: imaging, treatment and treatment outcomes. Oral Oncol. 2008;44:211–215. - PubMed
-
- Audrey RCB. Head and Neck: Squamous cell carcinoma: an overview. Atlas Genet Cytogenet Oncol Haematol. 2012;16:145–155.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

