Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway
- PMID: 26934446
- PMCID: PMC4951278
- DOI: 10.18632/oncotarget.7776
Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway
Abstract
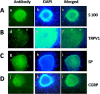
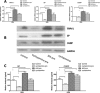
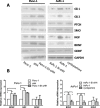
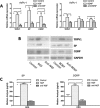
Abdominal pain is a critical clinical symptom in pancreatic cancer (PC) that affects the quality of life for PC patients. However, the pathogenesis of PC pain is largely unknown. In this study, we show that PC pain is initiated by the sonic hedgehog (sHH) signaling pathway in pancreatic stellate cells (PSCs), which is activated by sHH secreted from PC cells, and then, neurotrophic factors derived from PSCs mediate the pain. The different culture systems were established in vitro, and the expression of sHH pathway molecules, neurotrophic factors, TRPV1, and pain factors were examined. Capsaicin-evoked TRPV1 currents in dorsal root ganglion (DRG) neurons were examined by the patch-clamp technique. Pain-related behavior was observed in an orthotopic tumor model. sHH and PSCs increased the expression and secretion of TRPV1, SP, and CGRP by inducing NGF and BDNF in a co-culture system, also increasing TRPV1 current. But, suppressing sHH pathway or NGF reduced the expression of TRPV1, SP, and CGRP. In vivo, PSCs and PC cells that expressed high levels of sHH could enhance pain behavior. Furthermore, the blockade of NGF or TRPV1 significantly attenuated the pain response to mechanical stimulation compared with the control. Our results demonstrate that sHH signaling pathway is involved in PC pain, and PSCs play an essential role in the process greatly by inducing NGF.
Keywords: pain; pancreatic cancer; pancreatic stellate cells; sHH.
Conflict of interest statement
The authors declare that there is no conflicts of interest related to this work.
Figures



Similar articles
-
Sonic Hedgehog Signaling Pathway: A Role in Pain Processing.Neurochem Res. 2023 Jun;48(6):1611-1630. doi: 10.1007/s11064-023-03864-5. Epub 2023 Feb 4. Neurochem Res. 2023. PMID: 36738366 Review.
-
Sonic hedgehog signaling pathway promotes pancreatic cancer pain via nerve growth factor.Reg Anesth Pain Med. 2020 Feb;45(2):137-144. doi: 10.1136/rapm-2019-100991. Epub 2019 Dec 1. Reg Anesth Pain Med. 2020. PMID: 31792027
-
Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer.Clin Cancer Res. 2014 Aug 15;20(16):4326-38. doi: 10.1158/1078-0432.CCR-13-3426. Epub 2014 Jun 19. Clin Cancer Res. 2014. PMID: 24947933
-
Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion.BMC Neurosci. 2005 Jan 24;6:4. doi: 10.1186/1471-2202-6-4. BMC Neurosci. 2005. PMID: 15667652 Free PMC article.
-
Thiosemicarbazones reprogram pancreatic cancer bidirectional oncogenic signaling between cancer cells and stellate cells to suppress desmoplasia.Future Med Chem. 2022 Jul;14(13):1005-1017. doi: 10.4155/fmc-2022-0050. Epub 2022 Jun 7. Future Med Chem. 2022. PMID: 35670251 Review.
Cited by
-
Targeting HGF/c-MET Axis in Pancreatic Cancer.Int J Mol Sci. 2020 Dec 1;21(23):9170. doi: 10.3390/ijms21239170. Int J Mol Sci. 2020. PMID: 33271944 Free PMC article. Review.
-
Animal Models of Cancer-Related Pain: Current Perspectives in Translation.Front Pharmacol. 2020 Nov 26;11:610894. doi: 10.3389/fphar.2020.610894. eCollection 2020. Front Pharmacol. 2020. PMID: 33381048 Free PMC article. Review.
-
Sonic Hedgehog Signaling Pathway: A Role in Pain Processing.Neurochem Res. 2023 Jun;48(6):1611-1630. doi: 10.1007/s11064-023-03864-5. Epub 2023 Feb 4. Neurochem Res. 2023. PMID: 36738366 Review.
-
Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance.Cancers (Basel). 2022 Nov 24;14(23):5793. doi: 10.3390/cancers14235793. Cancers (Basel). 2022. PMID: 36497277 Free PMC article. Review.
-
Biology of pancreatic stellate cells-more than just pancreatic cancer.Pflugers Arch. 2017 Sep;469(9):1039-1050. doi: 10.1007/s00424-017-1968-0. Epub 2017 Apr 5. Pflugers Arch. 2017. PMID: 28382480 Free PMC article. Review.
References
-
- Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. - PubMed
-
- Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, Muller MW, Giese T, Buchler MW, Giese NA, Friess H. Pancreatic neuropathy and neuropathic pain—a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186 e171. - PubMed
-
- Anaparthy R, Pasricha PJ. Pain and chronic pancreatitis: is it the plumbing or the wiring? Curr Gastroenterol Rep. 2008;10:101–106. - PubMed
-
- Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004;321:219–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

