Genomic characterization of patient-derived xenograft models established from fine needle aspirate biopsies of a primary pancreatic ductal adenocarcinoma and from patient-matched metastatic sites
- PMID: 26934555
- PMCID: PMC4941373
- DOI: 10.18632/oncotarget.7718
Genomic characterization of patient-derived xenograft models established from fine needle aspirate biopsies of a primary pancreatic ductal adenocarcinoma and from patient-matched metastatic sites
Abstract
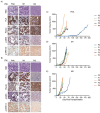
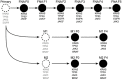
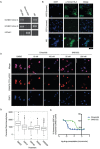
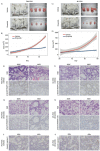
N-of-1 trials target actionable mutations, yet such approaches do not test genomically-informed therapies in patient tumor models prior to patient treatment. To address this, we developed patient-derived xenograft (PDX) models from fine needle aspiration (FNA) biopsies (FNA-PDX) obtained from primary pancreatic ductal adenocarcinoma (PDAC) at the time of diagnosis. Here, we characterize PDX models established from one primary and two metastatic sites of one patient. We identified an activating KRAS G12R mutation among other mutations in these models. In explant cells derived from these PDX tumor models with a KRAS G12R mutation, treatment with inhibitors of CDKs (including CDK9) reduced phosphorylation of a marker of CDK9 activity (phospho-RNAPII CTD Ser2/5) and reduced viability/growth of explant cells derived from PDAC PDX models. Similarly, a CDK inhibitor reduced phospho-RNAPII CTD Ser2/5, increased apoptosis, and inhibited tumor growth in FNA-PDX and patient-matched metastatic-PDX models. In summary, PDX models can be constructed from FNA biopsies of PDAC which in turn can enable genomic characterization and identification of potential therapies.
Keywords: CDK9; KRAS; fine needle aspirate biopsy; pancreatic ductal adenocarcinoma; patient-derived xenograft.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg P V, Kuramochi H, Tanaka K, Singh S, Salimi-Moosavi H, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–61. - PubMed
-
- Feldmann G, Mishra A, Bisht S, Karikari C, Garrido-Laguna I, Rasheed Z, Ottenhof NA, Dadon T, Alvarez H, Fendrich V, Rajeshkumar N V, Matsui W, Brossart P, et al. Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol Ther. 2011;12:598–609. - PMC - PubMed
-
- Rubio-Viqueira B, Hidalgo M. Direct in vivo xenograft tumor model for predicting chemotherapeutic drug response in cancer patients. Clin Pharmacol Ther. 2009;85:217–21. - PubMed
-
- DeRose YS, Wang G, Lin Y-C, Bernard PS, Buys SS, Ebbert MTW, Factor R, Matsen C, Milash BA, Nelson E, Neumayer L, Randall RL, Stijleman IJ, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–20. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

