Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure
- PMID: 26934955
- PMCID: PMC4987841
- DOI: 10.1038/npp.2016.32
Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure
Abstract
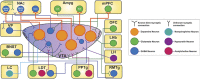
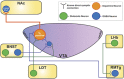
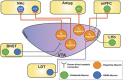
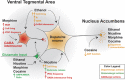
Addictive substances are known to increase dopaminergic signaling in the mesocorticolimbic system. The origin of this dopamine (DA) signaling originates in the ventral tegmental area (VTA), which sends afferents to various targets, including the nucleus accumbens, the medial prefrontal cortex, and the basolateral amygdala. VTA DA neurons mediate stimuli saliency and goal-directed behaviors. These neurons undergo robust drug-induced intrinsic and extrinsic synaptic mechanisms following acute and chronic drug exposure, which are part of brain-wide adaptations that ultimately lead to the transition into a drug-dependent state. Interestingly, recent investigations of the differential subpopulations of VTA DA neurons have revealed projection-specific functional roles in mediating reward, aversion, and stress. It is now critical to view drug-induced neuroadaptations from a circuit-level perspective to gain insight into how differential dopaminergic adaptations and signaling to targets of the mesocorticolimbic system mediates drug reward. This review hopes to describe the projection-specific intrinsic characteristics of these subpopulations, the differential afferent inputs onto these VTA DA neuron subpopulations, and consolidate findings of drug-induced plasticity of VTA DA neurons and highlight the importance of future projection-based studies of this system.
Figures

References
-
- Anstrom KK, Woodward DJ (2005). Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology 30: 1832–1840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

