Protective effect of grape seed extract against cadmium-induced testicular dysfunction
- PMID: 26935153
- PMCID: PMC4805107
- DOI: 10.3892/mmr.2016.4928
Protective effect of grape seed extract against cadmium-induced testicular dysfunction
Abstract
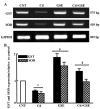
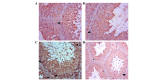
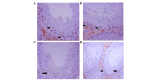
Cadmium (Cd) is the most prevalent toxic metal present in livestock feed; therefore, the present study aimed to examine the ameliorative effects of grape seed extract (GSE) on cadmium chloride (CdCl2)‑induced testicular dysfunction of Wistar rats. Male adult Wistar rats (40 rats; n=10/group) were divided into four equal groups. Group one was used as a control, and was given ad libitum access to food and water. Groups 2‑4 were treated with CdCl2 [5 mg/kg body weight (BW)], GSE (400 mg/kg BW, orally), and GSE plus CdCl2, respectively. Blood and testicular tissues were collected and assayed for biochemical and histopathological changes, respectively. Testicular genes were expressed using semi‑quantitative RT‑PCR analysis. The results of the present study demonstrated that there was a decrease in serum testosterone levels following CdCl2 toxicity, which were normalized after GSE co-administration. Furthermore, CdCl2 significantly increased the serum levels of malondialdehyde, and decreased levels of antioxidants. At the histopathological level, the testes of the CdCl2 group exhibited congestion, edema in the interstitial blood vessels, irregular arrangement of the epithelial lining of the seminiferous tubules, and degeneration and sloughing of the spermatogenic cells, which accumulated in the center of the seminiferous tubules. Such pathological alterations were ameliorated following treatment with GSE in the CdCl2 plus GSE group. The immunohistochemical expression of B‑cell lymphoma 2‑associated X protein was high in the CdCl2 group, and low in the control and GSE groups. Co‑treatment with GSE and CdCl2 exhibited ameliorative effects on the immunoreactivity of B‑cell lymphoma 2‑associated X protein. CdCl2 toxicity induced a significant downregulation in the mRNA expression levels of cytochrome P450 cholesterol side‑chain cleavage enzyme, cytochrome P450 17A1, 3β‑hydroxysteroid dehydrogenase (3β‑HSD), 17β‑HSD, androgen receptor, steroidogenic acute regulatory protein, and follicle‑stimulating hormone receptor. GSE administration exhibited a stimulatory effect on steroidogenesis‑associated enzymes, and co‑treatment with GSE and CdCl2 normalized and upregulated the mRNA expression levels of these examined genes. This study concluded that GSE has beneficial protective effects against the deleterious effects of CdCl2 on the testis.
Figures


References
-
- Goyer R. Toxic effect of metal: Casarett and doult's toxicology. In: Klaassen CD, editor. The Basic Science of Poisons. 5th edition. McGraw-Hill; New York: 1995. pp. 691–736.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

