Decoding the organization of spinal circuits that control locomotion
- PMID: 26935168
- PMCID: PMC4844028
- DOI: 10.1038/nrn.2016.9
Decoding the organization of spinal circuits that control locomotion
Abstract
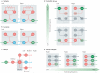
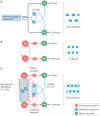
Unravelling the functional operation of neuronal networks and linking cellular activity to specific behavioural outcomes are among the biggest challenges in neuroscience. In this broad field of research, substantial progress has been made in studies of the spinal networks that control locomotion. Through united efforts using electrophysiological and molecular genetic network approaches and behavioural studies in phylogenetically diverse experimental models, the organization of locomotor networks has begun to be decoded. The emergent themes from this research are that the locomotor networks have a modular organization with distinct transmitter and molecular codes and that their organization is reconfigured with changes to the speed of locomotion or changes in gait.
Figures



References
-
- Drew T, Marigold DS. Taking the next step: cortical contributions to the control of locomotion. Curr. Opin. Neurobiol. 2015;33:25–33. - PubMed
-
- Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov. Disord. 2013;28:1483–1491. - PubMed
-
- Garcia-Rill E. The basal ganglia and the locomotor regions. Brain Res. 1986;396:47–63. - PubMed
-
- Grillner S, Robertson B. The basal ganglia downstream control of brainstem motor centres — an evolutionarily conserved strategy. Curr. Opin. Neurobiol. 2015;33:47–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

