Role of Alix in miRNA packaging during extracellular vesicle biogenesis
- PMID: 26935291
- PMCID: PMC4790646
- DOI: 10.3892/ijmm.2016.2488
Role of Alix in miRNA packaging during extracellular vesicle biogenesis
Abstract
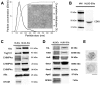
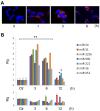
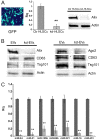
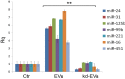
Evidence indicates that Alix, an accessory protein of the endosomal sorting complex required for transport (ESCRT), is involved in the biogenesis of extracellular vesicles (EVs). EVs contain selected patterns of microRNAs (miRNAs or miRs); however, little is known about the mechanisms of miRNA enrichment in EVs. The aim of the present study was to evaluate whether Alix is involved in the packaging of miRNAs within EVs released by human liver stem‑like cells (HLSCs). EVs released from HLSCs were enriched with miRNAs and expressed Alix and several RNA-binding proteins, including Argonaute 2 (Ago2), a member of the Argonaute family known to be involved in the transport and the processing of miRNAs. Co-immunoprecipitation experiments revealed an association between Alix and Ago2. The results from RT-qPCR indicated that in the Alix/Ago2 immunoprecipitates, miRNAs were detectable. EVs were instrumental in transferring selected miRNAs from HLSCs to human endothelial cells absent in the latter cells. Alix knockdown did not influence the number of EVs released by HLSCs, but it significantly decreased miRNA expression levels in the EVs and consequently their transfer to the endothelium. Our findings indicate that Alix binds to Ago2 and miRNAs, suggesting that it plays a key role in miRNA enrichment during EV biogenesis. These results may represent a novel function of Alix, demonstrating its involvement in the EV-mediated transfer of miRNAs.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

