Inflammation-Related IL1β/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma
- PMID: 26935421
- PMCID: PMC4854753
- DOI: 10.1158/1940-6207.CAPR-15-0347
Inflammation-Related IL1β/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma
Abstract
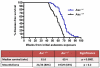
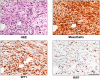
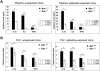
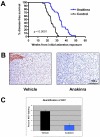
Exposure to asbestos is causally associated with the development of malignant mesothelioma, a cancer of cells lining the internal body cavities. Malignant mesothelioma is an aggressive cancer resistant to all current therapies. Once inhaled or ingested, asbestos causes inflammation in and around tissues that come in contact with these carcinogenic fibers. Recent studies suggest that inflammation is a major contributing factor in the development of many types of cancer, including malignant mesothelioma. The NALP3/NLRP3 inflammasome, including the component ASC, is thought to be an important mediator of inflammation in cells that sense extracellular insults, such as asbestos, and activate a signaling cascade resulting in release of mature IL1β and recruitment of inflammatory cells. To determine if inflammasome-mediated inflammation contributes to asbestos-induced malignant mesothelioma, we chronically exposed Asc-deficient mice and wild-type littermates to asbestos and evaluated differences in tumor incidence and latency. The Asc-deficient mice showed significantly delayed tumor onset and reduced malignant mesothelioma incidence compared with wild-type animals. We also tested whether inflammation-related release of IL1β contributes to tumor development in an accelerated mouse model of asbestos-induced malignant mesothelioma. Nf2(+/-);Cdkn2a(+/-) mice exposed to asbestos in the presence of anakinra, an IL1 receptor (IL1R) antagonist, showed a marked delay in the median time of malignant mesothelioma onset compared with similarly exposed mice given vehicle control (33.1 weeks vs. 22.6 weeks, respectively). Collectively, these studies provide evidence for a link between inflammation-related IL1β/IL1R signaling and the development of asbestos-induced malignant mesothelioma. Furthermore, these findings provide rationale for chemoprevention strategies targeting IL1β/IL1R signaling in high-risk, asbestos-exposed populations. Cancer Prev Res; 9(5); 406-14. ©2016 AACR.
©2016 American Association for Cancer Research.
Figures

References
-
- Burki T. Health experts concerned over India's asbestos industry. Lancet. 2010;375:626–7. - PubMed
-
- Flejter WL, Li FP, Antman KH, Testa JR. Recurring loss involving chromosomes 1, 3, and 22 in malignant mesothelioma: possible sites of tumor suppressor genes. Genes, Chromosomes Cancer. 1989;1:148–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

