Enhancing full-length antibody production by signal peptide engineering
- PMID: 26935575
- PMCID: PMC4776426
- DOI: 10.1186/s12934-016-0445-3
Enhancing full-length antibody production by signal peptide engineering
Abstract
Background: Protein secretion to the periplasm of Escherichia coli offers an attractive route for producing heterologous proteins including antibodies. In this approach, a signal peptide is fused to the N-terminus of the heterologous protein. The signal peptide mediates translocation of the heterologous protein from the cytoplasm to the periplasm and is cleaved during the translocation process. It was previously shown that optimization of the translation initiation region (TIR) which overlaps with the nucleotide sequence of the signal sequence improves the production of heterologous proteins. Despite the progress, there is still room to improve yields using secretion as a means to produce protein complexes such as full-length monoclonal antibodies (mAbs).
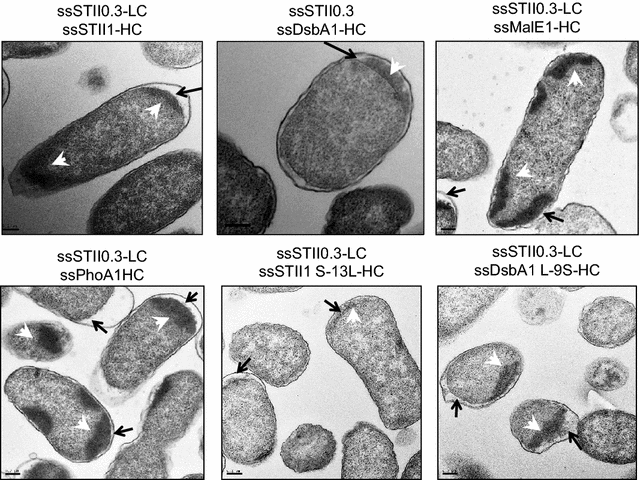
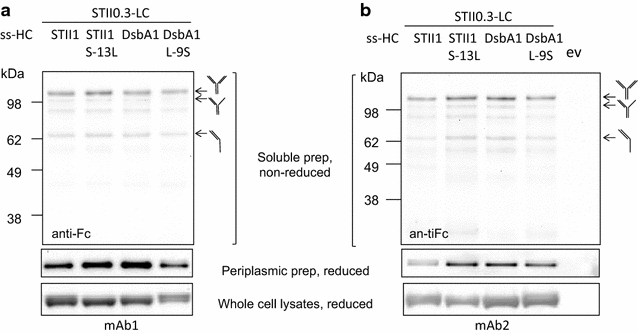
Results: In this study we identified the inefficient secretion of heavy chain as the limitation for full-length mAb accumulation in the periplasm. To improve heavy chain secretion we investigated the effects of various signal peptides at controlled TIR strengths. The signal peptide of disulfide oxidoreductase (DsbA) mediated more efficient secretion of heavy chain than the other signal peptides tested. Mutagenesis studies demonstrated that at controlled translational levels, hydrophobicity of the hydrophobic core (H-region) of the signal peptide is a critical factor for heavy chain secretion and full-length mAb accumulation in the periplasm. Increasing the hydrophobicity of a signal peptide enhanced heavy chain secretion and periplasmic levels of assembled full-length mAbs, while decreasing the hydrophobicity had the opposite effect.
Conclusions: This study demonstrates that under similar translational strengths, the hydrophobicity of the signal peptide plays an important role in heavy chain secretion. Increasing the hydrophobicity of the H-region and controlling TIR strengths can serve as an approach to improve heavy chain secretion and full-length mAb production in E. coli.
Figures



References
-
- Nossal NG, Heppel LA. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966;241:3055–3062. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

