Mechanism of intermediate filament recognition by plakin repeat domains revealed by envoplakin targeting of vimentin
- PMID: 26935805
- PMCID: PMC4782060
- DOI: 10.1038/ncomms10827
Mechanism of intermediate filament recognition by plakin repeat domains revealed by envoplakin targeting of vimentin
Abstract
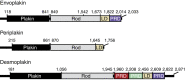
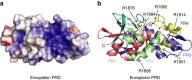
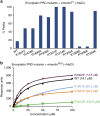
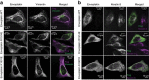
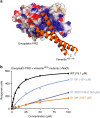
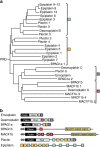
Plakin proteins form critical connections between cell junctions and the cytoskeleton; their disruption within epithelial and cardiac muscle cells cause skin-blistering diseases and cardiomyopathies. Envoplakin has a single plakin repeat domain (PRD) which recognizes intermediate filaments through an unresolved mechanism. Herein we report the crystal structure of envoplakin's complete PRD fold, revealing binding determinants within its electropositive binding groove. Four of its five internal repeats recognize negatively charged patches within vimentin via five basic determinants that are identified by nuclear magnetic resonance spectroscopy. Mutations of the Lys1901 or Arg1914 binding determinants delocalize heterodimeric envoplakin from intracellular vimentin and keratin filaments in cultured cells. Recognition of vimentin is abolished when its residues Asp112 or Asp119 are mutated. The latter slot intermediate filament rods into basic PRD domain grooves through electrosteric complementarity in a widely applicable mechanism. Together this reveals how plakin family members form dynamic linkages with cytoskeletal frameworks.
Figures
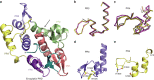
References
-
- Candi E., Schmidt R. & Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 (2005). - PubMed
-
- Sonnenberg A. & Liem R. K. Plakins in development and disease. Exp. Cell Res. 313, 2189–2203 (2007). - PubMed
-
- Huang Y., Li J. & Zhu X. Detection of anti-envoplakin and anti-periplakin autoantibodies by ELISA in patients with paraneoplastic pemphigus. Arch. Dermatol. Res. 301, 703–709 (2009). - PubMed
-
- Li J., Bu D. F., Huang Y. C. & Zhu X. J. Role of autoantibodies against the linker subdomains of envoplakin and periplakin in the pathogenesis of paraneoplastic pemphigus. Chin. Med. J. (Engl.) 122, 486–495 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

