Fusion of Enveloped Viruses in Endosomes
- PMID: 26935856
- PMCID: PMC4866878
- DOI: 10.1111/tra.12389
Fusion of Enveloped Viruses in Endosomes
Abstract
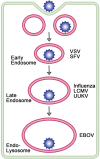
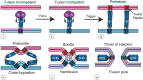
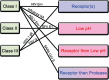
Ari Helenius launched the field of enveloped virus fusion in endosomes with a seminal paper in the Journal of Cell Biology in 1980. In the intervening years, a great deal has been learned about the structures and mechanisms of viral membrane fusion proteins as well as about the endosomes in which different enveloped viruses fuse and the endosomal cues that trigger fusion. We now recognize three classes of viral membrane fusion proteins based on structural criteria and four mechanisms of fusion triggering. After reviewing general features of viral membrane fusion proteins and viral fusion in endosomes, we delve into three characterized mechanisms for viral fusion triggering in endosomes: by low pH, by receptor binding plus low pH and by receptor binding plus the action of a protease. We end with a discussion of viruses that may employ novel endosomal fusion-triggering mechanisms. A key take-home message is that enveloped viruses that enter cells by fusing in endosomes traverse the endocytic pathway until they reach an endosome that has all of the environmental conditions (pH, proteases, ions, intracellular receptors and lipid composition) to (if needed) prime and (in all cases) trigger the fusion protein and to support membrane fusion.
Keywords: enveloped virus; fuse; low pH; membrane; prime; proteases; trigger; viral fusion protein; virus entry; virus receptors.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Marsh M, Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol 1980;142:439–454. - PubMed
-
- Marsh M, Bolzau E, Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell 1983;32:931–940. - PubMed
-
- Simons K, Garoff H, Helenius A. How an animal virus gets into and out of its host cell. Sci Am 1982;246:58–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

