Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study
- PMID: 26935876
- PMCID: PMC5036253
- DOI: 10.1136/gutjnl-2015-311304
Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study
Abstract
Objective: The objective of this study was to assess the efficacy, safety and tolerability of vonoprazan, a novel potassium-competitive acid blocker, as a component of Helicobacter pylori eradication therapy.
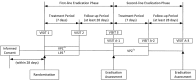
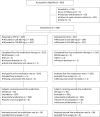
Design: A randomised, double-blind, multicentre, parallel-group study was conducted to verify the non-inferiority of vonoprazan 20 mg to lansoprazole 30 mg as part of first-line triple therapy (with amoxicillin 750 mg and clarithromycin 200 or 400 mg) in H pylori-positive patients with gastric or duodenal ulcer history. The first 50 patients failing first-line therapy with good compliance also received second-line vonoprazan-based triple therapy (with amoxicillin 750 mg and metronidazole 250 mg) as an open-label treatment.
Results: Of the 650 subjects randomly allocated to either first-line triple therapy, 641 subjects completed first-line therapy and 50 subjects completed second-line therapy. The first-line eradication rate (primary end point) was 92.6% (95% CI 89.2% to 95.2%) with vonoprazan versus 75.9% (95% CI 70.9% to 80.5%) with lansoprazole, with the difference being 16.7% (95% CI 11.2% to 22.1%) in favour of vonoprazan, thus confirming the non-inferiority of vonoprazan (p<0.0001). The second-line eradication rate (secondary end point) was also high (98.0%; 95% CI 89.4% to 99.9%) in those who received second-line therapy (n=50). Both first-line triple therapies were well tolerated with no notable differences. Second-line triple therapy was also well tolerated.
Conclusion: Vonoprazan is effective as part of first-line triple therapy and as part of second-line triple therapy in H pylori-positive patients with a history of gastric or duodenal ulcer.
Trial registration number: NCT01505127.
Keywords: ANTIBIOTIC THERAPY; CLINICAL TRIALS; HELICOBACTER PYLORI - TREATMENT; HELICOBACTER PYLORI INFECTION.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


Comment in
-
Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues.Gut. 2017 Feb;66(2):384-386. doi: 10.1136/gutjnl-2016-311796. Epub 2016 Apr 7. Gut. 2017. PMID: 27056876 Free PMC article. No abstract available.
-
Efficacy of vonoprazan-based second-line Helicobacter pylori eradication therapy in patients for whom vonoprazan-based first-line treatment failed.Gut. 2017 Apr;66(4):752-753. doi: 10.1136/gutjnl-2016-312028. Epub 2016 May 17. Gut. 2017. PMID: 27196582 No abstract available.
-
Reply to the letter by Dr Graham concerning ethical and interpretation issues with vonoprazan-containing H. pylori eradication therapy.Gut. 2017 Feb;66(2):386. doi: 10.1136/gutjnl-2016-312011. Epub 2016 May 3. Gut. 2017. PMID: 27196601 No abstract available.
-
In a world of increasing resistance emerges a hope to eradicate Helicobacter pylori: Vonoprazan.Turk J Gastroenterol. 2016 May;27(3):296-7. doi: 10.5152/tjg.2016.160002. Turk J Gastroenterol. 2016. PMID: 27210791 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
