The placebo response of injectable GLP-1 receptor agonists vs. oral DPP-4 inhibitors and SGLT-2 inhibitors: a systematic review and meta-analysis
- PMID: 26935973
- PMCID: PMC4917794
- DOI: 10.1111/bcp.12925
The placebo response of injectable GLP-1 receptor agonists vs. oral DPP-4 inhibitors and SGLT-2 inhibitors: a systematic review and meta-analysis
Abstract
Aims: The size of the placebo response in type 2 diabetes (T2DM) treatment and its relation to the route of drug administration have not been systematically reviewed. We aimed to determine weight loss, change in HbA1c and incidence of adverse events after treatment with injectable placebo GLP-1 receptor agonist (GLP-1ra), compared with oral placebo DPP-4 inhibitor (DPP-4i) and placebo SGLT-2 inhibitor (SGLT-2i).
Methods: PubMed, EMBASE and Central were searched up to September 2014 for randomized placebo controlled trials investigating GLP-1ra, DPP-4i or SGLT2-i. Data on placebo groups were extracted and pooled using a generic inverse variance random effects model.
Results: Sixty-seven trials were included, involving 2522, 5290 and 2028 patients randomized to placebo GLP-1ra, placebo DPP-4i and placebo SGLT-2i, respectively. Body weight decreased by -0.67 kg (95% CI -1.03, -0.31) after treatment with placebo GLP-1ra (-0.76 kg [95% CI -1.10, -0.43] with placebo short acting GLP-1ra and -0.32 kg [95% CI -1.75, 1.10] with placebo long acting GLP-1ra) and by -0.31 kg (95% CI -0.64, 0.01) with placebo DPP-4i (P = 0.06 for difference with placebo short acting GLP-1ra). Placebo SGLT-2i resulted in an intermediate -0.48 kg (95% CI -0.81, -0.15) weight loss. Weight loss with placebo showed a strong correlation with the active comparator drug (r(2) = 0.40-0.78). HbA1c changed little with placebo treatment (-0.23%, 0.10% and -0.13% for placebo GLP-1ra, DPP-4i and SGLT-2i). Adverse events occurred frequently with placebo, were often similar to the active comparator drug and led to drop-out in 2.0-2.7% of cases.
Conclusions: The response to placebo treatment was related to its active comparator, with injectable placebo GLP-1ra showing a relevant response on weight, whereas oral placebo DPP4i showed no significant response. These findings may suggest that subjective expectations influence T2DM treatment efficacy, which can possibly be employed therapeutically.
Keywords: meta-analysis; placebo effect; placebo response; type 2 diabetes.
© 2016 The British Pharmacological Society.
Figures

 ) placebo long acting GLP‐1ra; (
) placebo long acting GLP‐1ra; ( ) placebo short acting GLP‐1ra; (
) placebo short acting GLP‐1ra; ( ) placebo GLP‐1ra; (
) placebo GLP‐1ra; ( ) placebo DPP‐4i; (
) placebo DPP‐4i; ( ) placebo SGLT‐2i
) placebo SGLT‐2i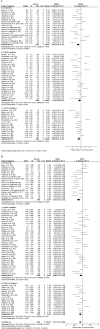
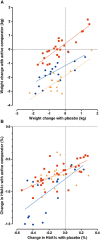
 ) GLP‐1ra (r
2 = 0.40); (
) GLP‐1ra (r
2 = 0.40); ( ) DPP‐4i (r
2 = 0.78); (
) DPP‐4i (r
2 = 0.78); ( ) SGLT‐2i (r
2 = 0.61); B) (
) SGLT‐2i (r
2 = 0.61); B) ( ) GLP‐1ra (r
2 = 0.42); (
) GLP‐1ra (r
2 = 0.42); ( ) DPP‐4i (r
2 = 0.50); (
) DPP‐4i (r
2 = 0.50); ( ) SGLT‐2i (r
2 = 0.07)
) SGLT‐2i (r
2 = 0.07)References
-
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001; 344: 1594–602. - PubMed
-
- Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta‐analysis of randomised controlled trials. Ann Rheum Dis 2008; 67: 1716–23. - PubMed
-
- Hauser W, Bartram‐Wunn E, Bartram C, Reinecke H, Tolle T. Systematic review: Placebo response in drug trials of fibromyalgia syndrome and painful peripheral diabetic neuropathy‐magnitude and patient‐related predictors. Pain 2011; 152: 1709–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

