Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament‑derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse
- PMID: 26936098
- PMCID: PMC4805094
- DOI: 10.3892/mmr.2016.4919
Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament‑derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse
Abstract
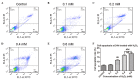
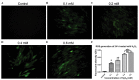
Pelvic organ prolapse (POP) is a global health problem, for which the pathophysiological mechanism remains to be fully elucidated. The loss of extracellular matrix protein has been considered to be the most important molecular basis facilitating the development of POP. Oxidative stress (OS) is a well‑recognized mechanism involved in fiber metabolic disorders. The present study aimed to clarify whether OS exists in the uterosacral ligament (USL) with POP, and to investigate the precise role of OS in collagen metabolism in human USL fibroblasts (hUSLFs). In the present study, 8‑hydroxyguanosine (8‑OHdG) and 4 hydroxynonenal (4‑HNE), as oxidative biomarkers, were examined by immunohistochemistry to evaluate oxidative injury in USL sections in POP (n=20) and non‑POP (n=20) groups. The primary cultured hUSLFs were treated with exogenous H2O2 to establish an original OS cell model, in which the expression levels of collagen, type 1, α1 (COL1A1), matrix metalloproteinase (MMP)‑2, tissue inhibitor of metalloproteinase (TIMP)‑2 and transforming growth factor (TGF)‑β1 were evaluated by western blot and reverse transcription‑quantitative polymerase chain reaction analyses. The results showed that the expression levels of 8‑OHdG and 4‑HNE in the POP group were significantly higher, compared with those in the control group. Collagen metabolism was regulated by H2O2 exposure in a concentration‑dependent manner, in which lower concentrations of H2O2 (0.1‑0.2 mM) stimulated the anabolism of COL1A1, whereas a higher concentration (0.4 mM) promoted catabolism. The expression levels of MMP‑2, TIMP‑2 and TGF‑β1 exhibited corresponding changes with the OS levels. These results suggested that OS may be involved in the pathophysiology of POP by contributing to collagen metabolic disorder in a severity‑dependent manner in hUSLFs, possibly through the regulation of MMPs, TIMPs and TGF‑β1 indirectly.
Figures




References
-
- Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646–651. - PubMed
-
- Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit. 2008;14:CR24–CR31. - PubMed
-
- Weber AM, Buchsbaum GM, Chen B, Clark AL, Damaser MS, Daneshgari F, Davis G, DeLancey J, Kenton K, Weidner AC, Word RA. Basic science and translational research in female pelvic floor disorders: Proceedings of an NIH-sponsored meeting. Neurourol Urodyn. 2004;23:288–301. doi: 10.1002/nau.20048. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

