Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution
- PMID: 26936099
- PMCID: PMC4820517
- DOI: 10.1128/CVI.00053-16
Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution
Abstract
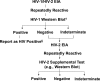
A concern during the early AIDS epidemic was the lack of a test to identify individuals who carried the virus. The first HIV antibody test, developed in 1985, was designed to screen blood products, not to diagnose AIDS. The first-generation assays detected IgG antibody and became positive 6 to 12 weeks postinfection. False-positive results occurred; thus, a two-test algorithm was developed using a Western blot or immunofluorescence test as a confirmatory procedure. The second-generation HIV test added recombinant antigens, and the third-generation HIV tests included IgM detection, reducing the test-negative window to approximately 3 weeks postinfection. Fourth- and fifth-generation HIV assays added p24 antigen detection to the screening assay, reducing the test-negative window to 11 to 14 days. A new algorithm addressed the fourth-generation assay's ability to detect both antibody and antigen and yet not differentiate between them. The fifth-generation HIV assay provides separate antigen and antibody results and will require yet another algorithm. HIV infection may now be detected approximately 2 weeks postexposure, with a reduced number of false-positive results.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Pear R. 1985. AIDS blood test to be available in 2 to 6 weeks. http://www.nytimes.com/1985/03/03/us/aids-blood-test-to-be-available-in-... Accessed 26 January 2016.
-
- Selik RM, Mokotoff ED, Branson B, Owen SM, Whitmore S, Hall HI. 2014. Revised surveillance case definition for HIV infection—United States, 2014. MMWR Morb Recommend Rep 63(RR-03):1–10. - PubMed
-
- Gallo RC, Sarin PS, Gelmann EP, Robert-Guroff M, Richardson E, Kalyanaraman VS, Mann D, Sidhu GD, Stahl RE, Zolla-Pazner S, Leibowitch J, Popovic M. 1983. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science 220:865–867. doi:10.1126/science.6601823. - DOI - PubMed
-
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnie L. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868–871. doi:10.1126/science.6189183. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

