Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers
- PMID: 26936519
- PMCID: PMC4850750
- DOI: 10.1111/dom.12654
Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers
Abstract
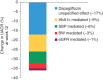
Aims: To characterize the effect of dapagliflozin on albuminuria and estimated glomerular filtration rate (eGFR) and to determine whether effects on albuminuria were mediated through changes in glycated haemoblogin (HbA1c), systolic blood pressure (SBP), body weight or eGFR.
Methods: We conducted a post hoc analysis of data pooled from two phase III clinical trials in hypertensive patients with type 2 diabetes (T2DM) on stable angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy, randomly assigned to dapagliflozin 10 mg/day or matched placebo. This analysis included only patients with microalbuminuria or macroalbuminuria at baseline.
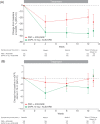
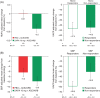
Results: Patients were randomized to receive dapagliflozin 10 mg (n = 167) or placebo (n = 189). Dapagliflozin resulted in greater 12-week reductions in albuminuria compared with placebo: -33.2% [95% confidence interval (CI) -45.4, -18.2]. The reduction in albuminuria was also present after adjusting for age, sex and changes in HbA1c, SBP, body weight and eGFR: -23.5% (95% CI -37.6, -6.3). There was a decrease in eGFR with dapagliflozin versus placebo that was readily reversed 1 week after last dose. No serious renal-related adverse events were observed in any group.
Conclusions: Dapagliflozin was effective in lowering albuminuria in patients with T2DM and hypertension using renin-angiotensin system blockade therapy. Reductions in albuminuria were still present after adjusting for changes in HbA1c, SBP, body weight and eGFR. Dapagliflozin-induced improvements in glycaemic control and reductions in SBP, coupled with other potentially beneficial renal effects, may lead to a reduced long-term renal and cardiovascular risk.
Keywords: albuminuria; dapagliflozin; diabetes; hypertension; sodium glucose cotransporter-2.
© 2016 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
References
-
- Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010; 375: 2223–2233. - PubMed
-
- Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2009; 85: 513–519. - PubMed
-
- Komoroski B, Vachharajani N, Boulton D et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose‐dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009; 85: 520–526. - PubMed
-
- Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med 2013; 125: 181–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

