C1q-targeted inhibition of the classical complement pathway prevents injury in a novel mouse model of acute motor axonal neuropathy
- PMID: 26936605
- PMCID: PMC4776408
- DOI: 10.1186/s40478-016-0291-x
C1q-targeted inhibition of the classical complement pathway prevents injury in a novel mouse model of acute motor axonal neuropathy
Abstract
Introduction: Guillain-Barré syndrome (GBS) is an autoimmune disease that results in acute paralysis through inflammatory attack on peripheral nerves, and currently has limited, non-specific treatment options. The pathogenesis of the acute motor axonal neuropathy (AMAN) variant is mediated by complement-fixing anti-ganglioside antibodies that directly bind and injure the axon at sites of vulnerability such as nodes of Ranvier and nerve terminals. Consequently, the complement cascade is an attractive target to reduce disease severity. Recently, C5 complement component inhibitors that block the formation of the membrane attack complex and subsequent downstream injury have been shown to be efficacious in an in vivo anti-GQ1b antibody-mediated mouse model of the GBS variant Miller Fisher syndrome (MFS). However, since gangliosides are widely expressed in neurons and glial cells, injury in this model was not targeted exclusively to the axon and there are currently no pure mouse models for AMAN. Additionally, C5 inhibition does not prevent the production of early complement fragments such as C3a and C3b that can be deleterious via their known role in immune cell and macrophage recruitment to sites of neuronal damage.
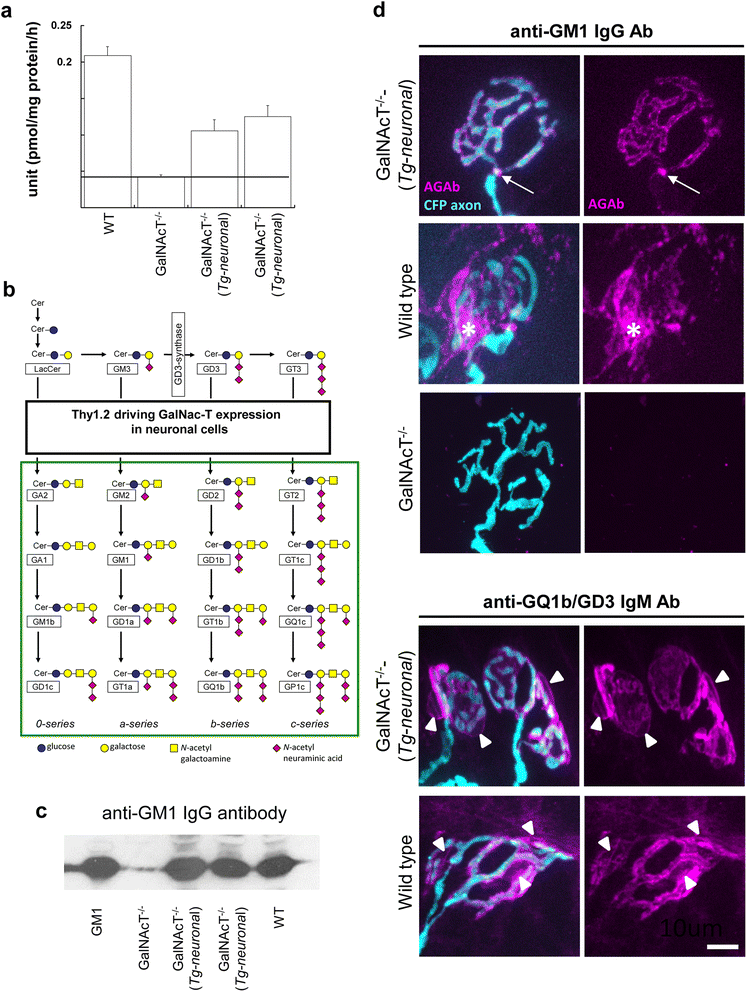
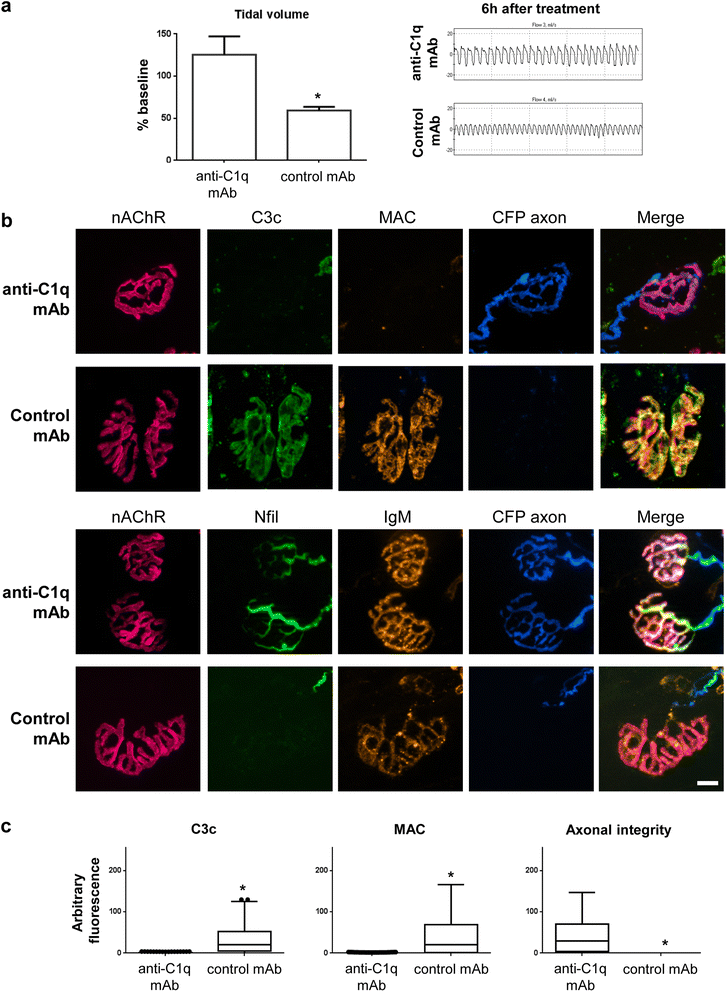
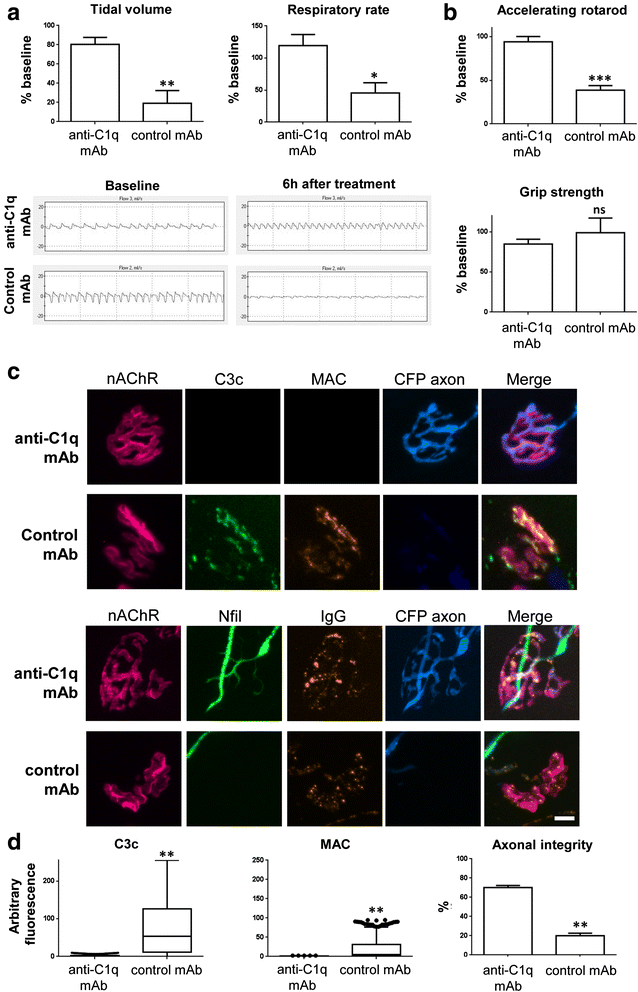
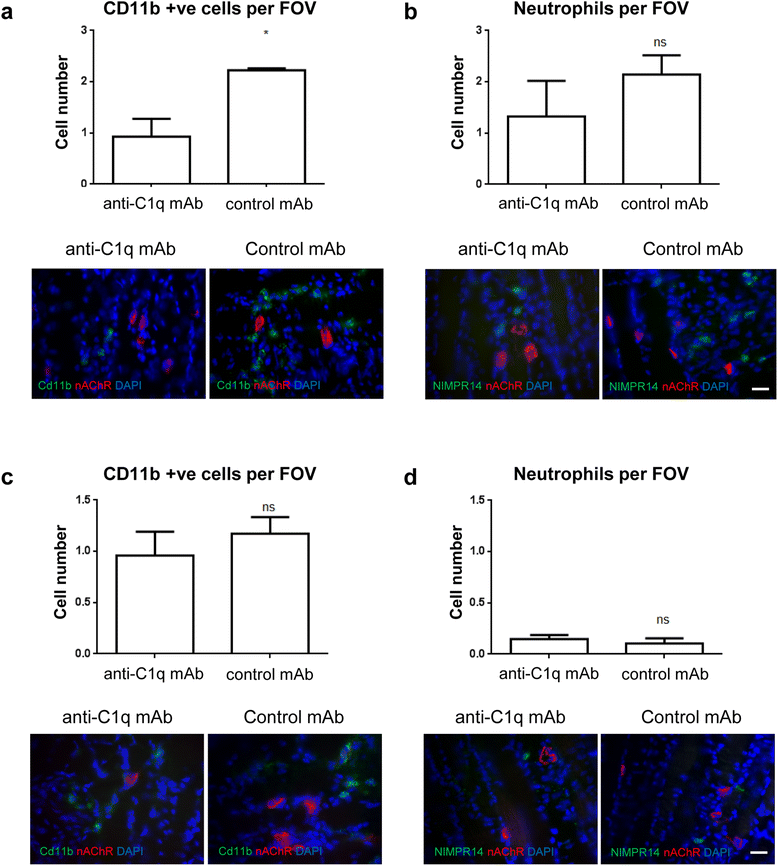
Results and conclusions: In this study, we first developed a new in vivo transgenic mouse model of AMAN using mice that express complex gangliosides exclusively in neurons, thereby enabling specific targeting of axons with anti-ganglioside antibodies. Secondly, we have evaluated the efficacy of a novel anti-C1q antibody (M1) that blocks initiation of the classical complement cascade, in both the newly developed anti-GM1 antibody-mediated AMAN model and our established MFS model in vivo. Anti-C1q monoclonal antibody treatment attenuated complement cascade activation and deposition, reduced immune cell recruitment and axonal injury, in both mouse models of GBS, along with improvement in respiratory function. These results demonstrate that neutralising C1q function attenuates injury with a consequent neuroprotective effect in acute GBS models and promises to be a useful new target for human therapy.
Figures
Similar articles
-
Eculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine model.Brain. 2008 May;131(Pt 5):1197-208. doi: 10.1093/brain/awm316. Epub 2008 Jan 8. Brain. 2008. PMID: 18184663
-
Anti-ganglioside antibody internalization attenuates motor nerve terminal injury in a mouse model of acute motor axonal neuropathy.J Clin Invest. 2012 Mar;122(3):1037-51. doi: 10.1172/JCI59110. Epub 2012 Feb 6. J Clin Invest. 2012. PMID: 22307327 Free PMC article.
-
The immunobiology of Guillain-Barré syndromes.J Peripher Nerv Syst. 2005 Jun;10(2):94-112. doi: 10.1111/j.1085-9489.2005.0010202.x. J Peripher Nerv Syst. 2005. PMID: 15958123 Review.
-
Complement inhibition prevents glial nodal membrane injury in a GM1 antibody-mediated mouse model.Brain Commun. 2022 Nov 23;4(6):fcac306. doi: 10.1093/braincomms/fcac306. eCollection 2022. Brain Commun. 2022. PMID: 36523267 Free PMC article.
-
Anti-ganglioside antibodies and the presynaptic motor nerve terminal.Ann N Y Acad Sci. 2008;1132:114-23. doi: 10.1196/annals.1405.010. Ann N Y Acad Sci. 2008. PMID: 18567860 Review.
Cited by
-
Guillain-Barré syndrome: a century of progress.Nat Rev Neurol. 2016 Dec;12(12):723-731. doi: 10.1038/nrneurol.2016.172. Epub 2016 Nov 18. Nat Rev Neurol. 2016. PMID: 27857121 Review.
-
Progress in Guillain-Barré syndrome immunotherapy-A narrative review of new strategies in recent years.Hum Vaccin Immunother. 2023 Aug 1;19(2):2215153. doi: 10.1080/21645515.2023.2215153. Hum Vaccin Immunother. 2023. PMID: 37278272 Free PMC article. Review.
-
Results From a Phase 1 Study Evaluating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of ANX005, a C1q Inhibitor, in Patients With Guillain-Barré Syndrome.J Peripher Nerv Syst. 2025 Mar;30(1):e70009. doi: 10.1111/jns.70009. J Peripher Nerv Syst. 2025. PMID: 40000167 Free PMC article. Clinical Trial.
-
Classical Complement Pathway Inhibition in a "Human-On-A-Chip" Model of Autoimmune Demyelinating Neuropathies.Adv Ther (Weinh). 2022 Jun;5(6):2200030. doi: 10.1002/adtp.202200030. Epub 2022 Apr 5. Adv Ther (Weinh). 2022. PMID: 36211621 Free PMC article.
-
The Neuroimmunology of Guillain-Barré Syndrome and the Potential Role of an Aging Immune System.Front Aging Neurosci. 2021 Jan 13;12:613628. doi: 10.3389/fnagi.2020.613628. eCollection 2020. Front Aging Neurosci. 2021. PMID: 33584245 Free PMC article. Review.
References
-
- Bowes T, Wagner ER, Boffey J, Nicholl D, Cochrane L, Benboubetra M, Conner J, Furukawa K, Willison HJ. Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain-Barre syndrome. Infect Immun. 2002;70:5008–18. - PMC - PubMed
-
- Bullens RW, O’Hanlon GM, Goodyear CS, Molenaar PC, Conner J, Willison HJ, Plomp JJ. Anti-GQ1b antibodies and evoked acetylcholine release at mouse motor endplates. Muscle Nerve. 2000;23:1035–43. - PubMed
-
- Court FA, Gillingwater TH, Melrose S, Sherman DL, Greenshields KN, Morton AJ, Harris JB, Willison HJ, Ribchester RR. Identity, developmental restriction and reactivity of extralaminar cells capping mammalian neuromuscular junctions. JCell Sci. 2008;121:3901–11. doi:10.1242/jcs.031047. - PubMed
-
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

