Advances in recombinant antibody manufacturing
- PMID: 26936774
- PMCID: PMC4803805
- DOI: 10.1007/s00253-016-7388-9
Advances in recombinant antibody manufacturing
Abstract
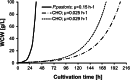
Since the first use of Chinese hamster ovary (CHO) cells for recombinant protein expression, production processes have steadily improved through numerous advances. In this review, we have highlighted several key milestones that have contributed to the success of CHO cells from the beginning of their use for monoclonal antibody (mAb) expression until today. The main factors influencing the yield of a production process are the time to accumulate a desired amount of biomass, the process duration, and the specific productivity. By comparing maximum cell densities and specific growth rates of various expression systems, we have emphasized the limiting parameters of different cellular systems and comprehensively described scientific approaches and techniques to improve host cell lines. Besides the quantitative evaluation of current systems, the quality-determining properties of a host cell line, namely post-translational modifications, were analyzed and compared to naturally occurring polyclonal immunoglobulin fractions from human plasma. In summary, numerous different expression systems for mAbs are available and also under scientific investigation. However, CHO cells are the most frequently investigated cell lines and remain the workhorse for mAb production until today.
Keywords: Chinese hamster ovary (CHO); Human embryonic kidney (HEK); Mammalian expression systems; Monoclonal antibody (mAb) manufacturing; NS0; Optimization strategies; PER.C6; Process advances.
Figures


References
-
- Benton T, Chen T, McEntee M, Fox B, King D, Crombie R, Thomas TC, Bebbington C. The use of UCOE vectors in combination with a preadapted serum free, suspension cell line allows for rapid production of large quantities of protein. Cytotechnology. 2002;38:43–46. doi: 10.1023/A:1021141712344. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

