Myeloid-Derived Suppressor Cell Survival and Function Are Regulated by the Transcription Factor Nrf2
- PMID: 26936880
- PMCID: PMC4821672
- DOI: 10.4049/jimmunol.1501785
Myeloid-Derived Suppressor Cell Survival and Function Are Regulated by the Transcription Factor Nrf2
Abstract
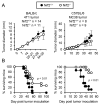
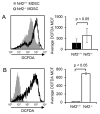
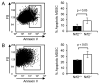
Tumor-induced myeloid-derived suppressor cells (MDSC) contribute to immune suppression in tumor-bearing individuals and are a major obstacle to effective immunotherapy. Reactive oxygen species (ROS) are one of the mechanisms used by MDSC to suppress T cell activation. Although ROS are toxic to most cells, MDSC survive despite their elevated content and release of ROS. NF erythroid 2-related factor 2 (Nrf2) is a transcription factor that regulates a battery of genes that attenuate oxidative stress. Therefore, we hypothesized that MDSC resistance to ROS may be regulated by Nrf2. To test this hypothesis, we used Nrf2(+/+)and Nrf2(-/-)BALB/c and C57BL/6 mice bearing 4T1 mammary carcinoma and MC38 colon carcinoma, respectively. Nrf2 enhanced MDSC suppressive activity by increasing MDSC production of H2O2, and it increased the quantity of tumor-infiltrating MDSC by reducing their oxidative stress and rate of apoptosis. Nrf2 did not affect circulating levels of MDSC in tumor-bearing mice because the decreased apoptotic rate of tumor-infiltrating MDSC was balanced by a decreased rate of differentiation from bone marrow progenitor cells. These results demonstrate that Nrf2 regulates the generation, survival, and suppressive potency of MDSC, and that a feedback homeostatic mechanism maintains a steady-state level of circulating MDSC in tumor-bearing individuals.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures


References
-
- Kawasaki Y, Ishigami S, Arigami T, Uenosono Y, Yanagita S, Uchikado Y, Kita Y, Nishizono Y, Okumura H, Nakajo A, Kijima Y, Maemura K, Natsugoe S. Clinicopathological significance of nuclear factor (erythroid-2)-related factor 2 (Nrf2) expression in gastric cancer. BMC Cancer. 2015;15:5. - PMC - PubMed
-
- Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158–4168. - PubMed
-
- Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N, Itoh K, Yamamoto M. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–3432. - PubMed
-
- Mohler J, Mahaffey JW, Deutsch E, Vani K. Control of Drosophila head segment identity by the bZIP homeotic gene cnc. Development. 1995;121:237–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

