Similar prevalence and magnitude of auditory-evoked and visually evoked activity in the frontal eye fields: implications for multisensory motor control
- PMID: 26936983
- PMCID: PMC4946597
- DOI: 10.1152/jn.00935.2015
Similar prevalence and magnitude of auditory-evoked and visually evoked activity in the frontal eye fields: implications for multisensory motor control
Abstract
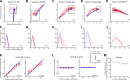
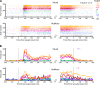
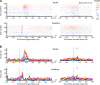
Saccadic eye movements can be elicited by more than one type of sensory stimulus. This implies substantial transformations of signals originating in different sense organs as they reach a common motor output pathway. In this study, we compared the prevalence and magnitude of auditory- and visually evoked activity in a structure implicated in oculomotor processing, the primate frontal eye fields (FEF). We recorded from 324 single neurons while 2 monkeys performed delayed saccades to visual or auditory targets. We found that 64% of FEF neurons were active on presentation of auditory targets and 87% were active during auditory-guided saccades, compared with 75 and 84% for visual targets and saccades. As saccade onset approached, the average level of population activity in the FEF became indistinguishable on visual and auditory trials. FEF activity was better correlated with the movement vector than with the target location for both modalities. In summary, the large proportion of auditory-responsive neurons in the FEF, the similarity between visual and auditory activity levels at the time of the saccade, and the strong correlation between the activity and the saccade vector suggest that auditory signals undergo tailoring to match roughly the strength of visual signals present in the FEF, facilitating accessing of a common motor output pathway.
Keywords: auditory; frontal eye field (FEF); multisensory; saccade.
Copyright © 2016 the American Physiological Society.
Figures






References
-
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci 24: 191–204, 1975.
-
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

