Dendritic spine remodeling following early and late Rac1 inhibition after spinal cord injury: evidence for a pain biomarker
- PMID: 26936986
- PMCID: PMC4922610
- DOI: 10.1152/jn.01057.2015
Dendritic spine remodeling following early and late Rac1 inhibition after spinal cord injury: evidence for a pain biomarker
Abstract
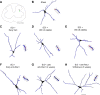
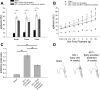
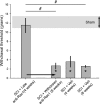
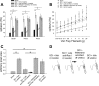
Neuropathic pain is a significant complication following spinal cord injury (SCI) with few effective treatments. Drug development for neuropathic pain often fails because preclinical studies do not always translate well to clinical conditions. Identification of biological characteristics predictive of disease state or drug responsiveness could facilitate more effective clinical translation. Emerging evidence indicates a strong correlation between dendritic spine dysgenesis and neuropathic pain. Because dendritic spines are located on dorsal horn neurons within the spinal cord nociceptive system, dendritic spine remodeling provides a unique opportunity to understand sensory dysfunction after SCI. In this study, we provide support for the postulate that dendritic spine profiles can serve as biomarkers for neuropathic pain. We show that dendritic spine profiles after SCI change to a dysgenic state that is characteristic of neuropathic pain in a Rac1-dependent manner. Suppression of the dysgenic state through inhibition of Rac1 activity is accompanied by attenuation of neuropathic pain. Both dendritic spine dysgenesis and neuropathic pain return when inhibition of Rac1 activity is lifted. These findings suggest the utility of dendritic spines as structural biomarkers for neuropathic pain.
Keywords: Rac1; biomarker; dendritic spines; pain; spinal cord injury.
Figures






References
-
- Arendt-Nielsen L, Eskehave TN, Egsgaard LL, Petersen KK, Graven-Nielsen T, Hoeck HC, Simonsen O, Siebuhr AS, Karsdal M, Bay-Jensen AC. Association between experimental pain biomarkers and serologic markers in patients with different degrees of painful knee osteoarthritis. Arthritis Rheum 66: 3317–3326, 2014. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21, 1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

