Impaired Serotonergic Brainstem Function during and after Seizures
- PMID: 26937010
- PMCID: PMC4879214
- DOI: 10.1523/JNEUROSCI.4331-15.2016
Impaired Serotonergic Brainstem Function during and after Seizures
Abstract
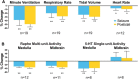
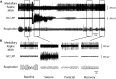
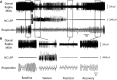
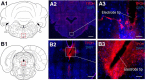
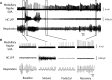
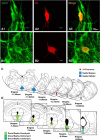
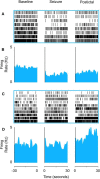
Impaired breathing, cardiac function, and arousal during and after seizures are important causes of morbidity and mortality. Previous work suggests that these changes are associated with depressed brainstem function in the ictal and post-ictal periods. Lower brainstem serotonergic systems are postulated to play an important role in cardiorespiratory changes during and after seizures, whereas upper brainstem serotonergic and other systems regulate arousal. However, direct demonstration of seizure-associated neuronal activity changes in brainstem serotonergic regions has been lacking. Here, we performed multiunit and single-unit recordings from medullary raphe and midbrain dorsal raphe nuclei in an established rat seizure model while measuring changes in breathing rate and depth as well as heart rate. Serotonergic neurons were identified by immunohistochemistry. Respiratory rate, tidal volume, and minute ventilation were all significantly decreased during and after seizures in this model. We found that population firing of neurons in the medullary and midbrain raphe on multiunit recordings was significantly decreased during the ictal and post-ictal periods. Single-unit recordings from identified serotonergic neurons in the medullary raphe revealed highly consistently decreased firing during and after seizures. In contrast, firing of midbrain raphe serotonergic neurons was more variable, with a mixture of increases and decreases. The markedly suppressed firing of medullary serotonergic neurons supports their possible role in simultaneously impaired cardiorespiratory function in seizures. Decreased arousal likely arises from depressed population activity of several neuronal pools in the upper brainstem and forebrain. These findings have important implications for preventing morbidity and mortality in people living with epilepsy.
Significance statement: Seizures often cause impaired breathing, cardiac dysfunction, and loss of consciousness. The brainstem and, specifically, brainstem serotonin neurons are thought to play an important role in controlling breathing, cardiac function, and arousal. We used an established rat seizure model to study the overall neuronal activity in the brainstem as well as firing of specific serotonin neurons while measuring cardiorespiratory function. Our results demonstrated overall decreases in brainstem neuronal activity and marked downregulation of lower brainstem serotonin neuronal firing in association with decreased breathing and heart rate during and after seizures. These findings point the way toward new treatments to augment brainstem function and serotonin, aiming to prevent seizure complications and reduce morbidity and mortality in people living with epilepsy.
Keywords: SUDEP; brainstem; consciousness; respiratory; serotonin; temporal lobe epilepsy.
Copyright © 2016 the authors 0270-6474/16/362712-12$15.00/0.
Figures

References
-
- Blessing WW. The lower brainstem and bodily homeostasis. Oxford: OUP; 1997.
-
- Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004a;63:1015–1021. doi: 10.1212/01.WNL.0000141086.91077.CD. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
