PrPSc-Specific Antibody Reveals C-Terminal Conformational Differences between Prion Strains
- PMID: 26937029
- PMCID: PMC4859706
- DOI: 10.1128/JVI.00088-16
PrPSc-Specific Antibody Reveals C-Terminal Conformational Differences between Prion Strains
Abstract
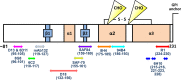
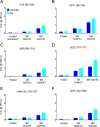
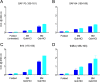
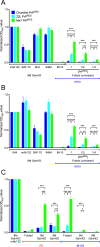
Understanding the structure of PrP(Sc) and its strain variation has been one of the major challenges in prion disease biology. To study the strain-dependent conformations of PrP(Sc), we purified proteinase-resistant PrP(Sc) (PrP(RES)) from mouse brains with three different murine-adapted scrapie strains (Chandler, 22L, and Me7) and systematically tested the accessibility of epitopes of a wide range of anti-PrP and anti-PrP(Sc) specific antibodies by indirect enzyme-linked immunosorbent assay (ELISA). We found that epitopes of most anti-PrP antibodies were hidden in the folded structure of PrP(RES), even though these epitopes are revealed with guanidine denaturation. However, reactivities to a PrP(Sc)-specific conformational C-terminal antibody showed significant differences among the three different prion strains. Our results provide evidence for strain-dependent conformational variation near the C termini of molecules within PrP(Sc) multimers.
Importance: It has long been apparent that prion strains can have different conformations near the N terminus of the PrP(Sc) protease-resistant core. Here, we show that a C-terminal conformational PrP(Sc)-specific antibody reacts differently to three murine-adapted scrapie strains. These results suggest, in turn, that conformational differences in the C terminus of PrP(Sc) also contribute to the phenotypic distinction between prion strains.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

