The Human Cytomegalovirus UL116 Gene Encodes an Envelope Glycoprotein Forming a Complex with gH Independently from gL
- PMID: 26937030
- PMCID: PMC4859709
- DOI: 10.1128/JVI.02517-15
The Human Cytomegalovirus UL116 Gene Encodes an Envelope Glycoprotein Forming a Complex with gH Independently from gL
Abstract
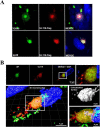
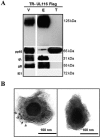
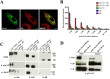
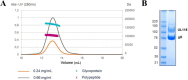
Human cytomegalovirus (HCMV) is a major cause of morbidity and mortality in transplant patients and is the leading viral cause of birth defects after congenital infection. HCMV infection relies on the recognition of cell-specific receptors by one of the viral envelope glycoprotein complexes. Either the gH/gL/gO or the gH/gL/UL128/UL130/UL131A (Pentamer) complex has been found to fulfill this role, accounting for HCMV entry into almost all cell types. We have studied the UL116 gene product, a putative open reading frame identified by in silico analysis and predicted to code for a secreted protein. Virus infection experiments in mammalian cells demonstrated that UL116 is expressed late in the HCMV replication cycle and is a heavily glycosylated protein that first localizes to the cellular site of virus assembly and then inserts into the virion envelope. Transient-transfection studies revealed that UL116 is efficiently transported to the plasma membrane when coexpressed with gH and that gL competes with UL116 for gH binding. Further evidence for gH/UL116 complex formation was obtained by coimmunoprecipitation experiments on both transfected and infected cells and biochemical characterization of the purified complex. In summary, our results show that the product of the UL116 gene is an HCMV envelope glycoprotein that forms a novel gH-based complex alternative to gH/gL. Remarkably, the gH/UL116 complex is the first herpesvirus gH-based gL-less complex.
Importance: HCMV infection can cause severe disease in immunocompromised adults and infants infected in utero The dissection of the HCMV entry machinery is important to understand the mechanism of viral infection and to identify new vaccine antigens. The gH/gL/gO and gH/gL/UL128/UL130/UL131 (Pentamer) complexes play a key role in HCMV cell entry and tropism. Both complexes are formed by an invariant gH/gL scaffold on which the other subunits assemble. Here, we show that the UL116 gene product is expressed in infected cells and forms a heterodimer with gH. The gH/UL116 complex is carried on the infectious virions, although in smaller amounts than gH/gL complexes. No gH/UL116/gL ternary complex formed in transfected cells, suggesting that the gH/UL116 complex is independent from gL. This new gH-based gL-free complex represents a potential target for a protective HCMV vaccine and opens new perspectives on the comprehension of the HCMV cell entry mechanism and tropism.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Mocarski JE, Shenk T, Griffiths PD, Pass RF. 2013. Cytomegaloviruses, p 1960–2014. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Britt W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Topics Microbiol Immunol 325:417–470. - PubMed
-
- Stratton KR, Durch JS, Lawrence RS. 2001. Vaccines for the 21st century: a tool for a decisionmaking. National Academy Press, Washington, DC. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

