Effects of Fecal Microbial Transplantation on Microbiome and Immunity in Simian Immunodeficiency Virus-Infected Macaques
- PMID: 26937040
- PMCID: PMC4859719
- DOI: 10.1128/JVI.00099-16
Effects of Fecal Microbial Transplantation on Microbiome and Immunity in Simian Immunodeficiency Virus-Infected Macaques
Abstract
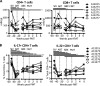
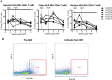
An altered intestinal microbiome during chronic human immunodeficiency virus (HIV) infection is associated with mucosal dysfunction, inflammation, and disease progression. We performed a preclinical evaluation of the safety and efficacy of fecal microbiota transplantation (FMT) as a potential therapeutic in HIV-infected individuals. Antiretroviral-treated, chronically simian immunodeficiency virus (SIV)-infected rhesus macaques received antibiotics followed by FMT. The greatest microbiota shift was observed after antibiotic treatment. The bacterial community composition at 2 weeks post-FMT resembled the pre-FMT community structure, although differences in the abundances of minor bacterial populations remained. Immunologically, we observed significant increases in the number of peripheral Th17 and Th22 cells and reduced CD4(+) T cell activation in gastrointestinal tissues post-FMT. Importantly, the transplant was well tolerated with no negative clinical side effects. Although this pilot study did not control for the differential contributions of antibiotic treatment and FMT to the observed results, the data suggest that FMT may have beneficial effects that should be further evaluated in larger studies.
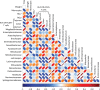
Importance: Due to the immunodeficiency and chronic inflammation that occurs during HIV infection, determination of the safety of FMT is crucial to prevent deleterious consequences if it is to be used as a treatment in the future. Here we used the macaque model of HIV infection and performed FMT on six chronically SIV-infected rhesus macaques on antiretroviral treatment. In addition to providing a preclinical demonstration of the safety of FMT in primates infected with a lentivirus, this study provided a unique opportunity to examine the relationships between alterations to the microbiome and immunological parameters. In this study, we found increased numbers of Th17 and Th22 cells as well as decreased activation of CD4(+) T cells post-FMT, and these changes correlated most strongly across all sampling time points with lower-abundance taxonomic groups and other taxonomic groups in the colon. Overall, these data provide evidence that changes in the microbiome, particularly in terms of diversity and changes in minor populations, can enhance immunity and do not have adverse consequences.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC, INSIGHT SMART Study Group. 2011. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 203:780–790. doi: 10.1093/infdis/jiq118. - DOI - PMC - PubMed
-
- Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM. 2014. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 210:1228–1238. doi: 10.1093/infdis/jiu238. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

