Order-Order Morphological Transitions for Dual Stimulus Responsive Diblock Copolymer Vesicles
- PMID: 26937051
- PMCID: PMC4762544
- DOI: 10.1021/acs.macromol.5b02470
Order-Order Morphological Transitions for Dual Stimulus Responsive Diblock Copolymer Vesicles
Abstract
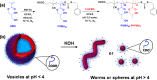
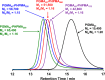
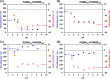
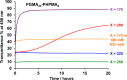
A series of non-ionic poly(glycerol monomethacrylate)-poly(2-hydroxypropyl methacrylate) (PGMA-PHPMA) diblock copolymer vesicles has been prepared by reversible addition-fragmentation chain transfer (RAFT) aqueous dispersion polymerization of HPMA at 70 °C at low pH using a carboxylic acid-based chain transfer agent. The degree of polymerization (DP) of the PGMA block was fixed at 43, and the DP of the PHPMA block was systematically varied from 175 to 250 in order to target vesicle phase space. Based on our recent work describing the analogous PGMA-PHPMA diblock copolymer worms [Lovett J. R.; Angew. Chem.2015, 54, 1279-1283], such diblock copolymer vesicles were expected to undergo an order-order morphological transition via ionization of the carboxylic acid end-group on switching the solution pH. Indeed, irreversible vesicle-to-sphere and vesicle-to-worm transitions were observed for PHPMA DPs of 175 and 200, respectively, as judged by turbidimetry, transmission electron microscopy (TEM), and dynamic light scattering (DLS) studies. However, such morphological transitions are surprisingly slow, with relatively long time scales (hours) being required at 20 °C. Moreover, no order-order morphological transitions were observed for vesicles comprising longer membrane-forming blocks (e.g., PGMA43-PHPMA225-250) on raising the pH from pH 3.5 to pH 6.0. However, in such cases the application of a dual stimulus comprising the same pH switch immediately followed by cooling from 20 to 5 °C, induces an irreversible vesicle-to-sphere transition. Finally, TEM and DLS studies conducted in the presence of 100 mM KCl demonstrated that the pH-responsive behavior arising from end-group ionization could be suppressed in the presence of added electrolyte. This is because charge screening suppresses the subtle change in the packing parameter required to drive the morphological transition.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
pH-responsive non-ionic diblock copolymers: ionization of carboxylic acid end-groups induces an order-order morphological transition.Angew Chem Int Ed Engl. 2015 Jan 19;54(4):1279-83. doi: 10.1002/anie.201409799. Epub 2014 Nov 21. Angew Chem Int Ed Engl. 2015. PMID: 25418214 Free PMC article.
-
Using Dynamic Covalent Chemistry To Drive Morphological Transitions: Controlled Release of Encapsulated Nanoparticles from Block Copolymer Vesicles.J Am Chem Soc. 2017 Jun 7;139(22):7616-7623. doi: 10.1021/jacs.7b02642. Epub 2017 May 23. J Am Chem Soc. 2017. PMID: 28497960 Free PMC article.
-
Critical Dependence of Molecular Weight on Thermoresponsive Behavior of Diblock Copolymer Worm Gels in Aqueous Solution.Macromolecules. 2018 Nov 13;51(21):8357-8371. doi: 10.1021/acs.macromol.8b01617. Epub 2018 Oct 16. Macromolecules. 2018. PMID: 30449901 Free PMC article.
-
Stimulus-responsive block copolymer nano-objects and hydrogels via dynamic covalent chemistry.Polym Chem. 2017 Sep 21;8(35):5374-5380. doi: 10.1039/c7py01242j. Epub 2017 Jul 28. Polym Chem. 2017. PMID: 29308094 Free PMC article.
-
Shape-Shifting Thermoresponsive Block Copolymer Nano-Objects.J Colloid Interface Sci. 2023 Mar 15;634:906-920. doi: 10.1016/j.jcis.2022.12.080. Epub 2022 Dec 18. J Colloid Interface Sci. 2023. PMID: 36566636 Review.
Cited by
-
Effect of Hydrophilic Monomer Distribution on Self-Assembly of a pH-Responsive Copolymer: Spheres, Worms and Vesicles from a Single Copolymer Composition.Angew Chem Int Ed Engl. 2021 Feb 23;60(9):4925-4930. doi: 10.1002/anie.202010501. Epub 2020 Nov 9. Angew Chem Int Ed Engl. 2021. PMID: 32997426 Free PMC article.
-
RAFT-mediated polymerization-induced self-assembly (RAFT-PISA): current status and future directions.Chem Sci. 2022 Mar 18;13(15):4192-4224. doi: 10.1039/d2sc00762b. eCollection 2022 Apr 13. Chem Sci. 2022. PMID: 35509470 Free PMC article. Review.
-
Histidine-Functionalized Diblock Copolymer Nanoparticles Exhibit Enhanced Adsorption onto Planar Stainless Steel.Macromol Rapid Commun. 2023 Aug;44(16):e2200903. doi: 10.1002/marc.202200903. Epub 2023 Jan 9. Macromol Rapid Commun. 2023. PMID: 36534428 Free PMC article.
-
Synthesis and Characterization of Charge-Stabilized Poly(4-hydroxybutyl acrylate) Latex by RAFT Aqueous Dispersion Polymerization: A New Precursor for Reverse Sequence Polymerization-Induced Self-Assembly.Macromolecules. 2023 Jun 2;56(11):4296-4306. doi: 10.1021/acs.macromol.3c00534. eCollection 2023 Jun 13. Macromolecules. 2023. PMID: 37333840 Free PMC article.
-
Synthesis of Highly Transparent Diblock Copolymer Vesicles via RAFT Dispersion Polymerization of 2,2,2-Trifluoroethyl Methacrylate in n-Alkanes.Macromolecules. 2021 Feb 9;54(3):1159-1169. doi: 10.1021/acs.macromol.0c02646. Epub 2021 Jan 22. Macromolecules. 2021. PMID: 33583957 Free PMC article.
References
-
- Antonietti M.; Förster S. Adv. Mater. 2003, 15, 1323–1333. 10.1002/adma.200300010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
