Reversing anticoagulant effects of novel oral anticoagulants: role of ciraparantag, andexanet alfa, and idarucizumab
- PMID: 26937198
- PMCID: PMC4762436
- DOI: 10.2147/VHRM.S89130
Reversing anticoagulant effects of novel oral anticoagulants: role of ciraparantag, andexanet alfa, and idarucizumab
Abstract
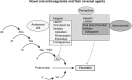
Novel oral anticoagulants (NOACs) are increasingly used in clinical practice, but lack of commercially available reversal agents is a major barrier for mainstream use of these therapies. Specific antidotes to NOACs are under development. Idarucizumab (aDabi-Fab, BI 655075) is a novel humanized mouse monoclonal antibody that binds dabigatran and reverses its anticoagulant effect. In a recent Phase III study (Reversal Effects of Idarucizumab on Active Dabigatran), a 5 g intravenous infusion of idarucizumab resulted in the normalization of dilute thrombin time in 98% and 93% of the two groups studied, with normalization of ecarin-clotting time in 89% and 88% patients. Two other antidotes, andexanet alfa (PRT064445) and ciraparantag (PER977) are also under development for reversal of NOACs. In this review, we discuss commonly encountered management issues with NOACs such as periprocedural management, laboratory monitoring of anticoagulation, and management of bleeding. We review currently available data regarding specific antidotes to NOACs with respect to pharmacology and clinical trials.
Keywords: dabigatran; idarucizumab; novel oral anticoagulant; reversal.
Figures
References
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. - PubMed
-
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. - PubMed
-
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

