Neoadjuvant therapy for early-stage breast cancer: the clinical utility of pertuzumab
- PMID: 26937204
- PMCID: PMC4762586
- DOI: 10.2147/CMAR.S55279
Neoadjuvant therapy for early-stage breast cancer: the clinical utility of pertuzumab
Abstract
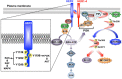
Approximately 20% of breast cancer patients harbor tumors that overexpress human epidermal growth factor receptor 2 (HER2; also known as ErbB2), a receptor tyrosine kinase that belongs to the epidermal growth factor receptor family of receptor tyrosine kinases. HER2 amplification and hyperactivation drive the growth and survival of breast cancers through the aberrant activation of proto-oncogenic signaling systems, particularly the Ras/MAP kinase and PI3K/AKT pathways. Although HER2-positive (HER2(+)) breast cancer was originally considered to be a highly aggressive form of the disease, the clinical landscape of HER2(+) breast cancers has literally been transformed by the approval of anti-HER2 agents for adjuvant and neoadjuvant settings. Indeed, pertuzumab is a novel monoclonal antibody that functions as an anti-HER2 agent by targeting the extracellular dimerization domain of the HER2 receptor; it is also the first drug to receive an accelerated approval by the US Food and Drug Administration for use in neoadjuvant settings in early-stage HER2(+) breast cancer. Here, we review the molecular and cellular factors that contribute to the pathophysiology of HER2 in breast cancer, as well as summarize the landmark preclinical and clinical findings underlying the approval and use of pertuzumab in the neoadjuvant setting. Finally, the molecular mechanisms operant in mediating resistance to anti-HER2 agents, and perhaps to pertuzumab as well, will be discussed, as will the anticipated clinical impact and future directions of pertuzumab in breast cancer patients.
Keywords: HER2; breast cancer; monoclonal antibody; neoadjuvant; pertuzumab; receptor tyrosine kinase; signal transduction; trastuzumab.
Figures

References
-
- Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast cancer: epidemiology and etiology. Cell Biochem Biophys. 2014 Dec 28; Epub. - PubMed
-
- Dittrich A, Gautrey H, Browell D, Tyson-Capper A. The HER2 signaling network in breast cancer-like a spider in its web. J Mammary Gland Biol Neoplasia. 2014;19(3–4):253–270. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. - PubMed
-
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–475. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

