Understanding Chemical versus Electrostatic Shifts in X-ray Photoelectron Spectra of Organic Self-Assembled Monolayers
- PMID: 26937264
- PMCID: PMC4761973
- DOI: 10.1021/acs.jpcc.5b12387
Understanding Chemical versus Electrostatic Shifts in X-ray Photoelectron Spectra of Organic Self-Assembled Monolayers
Abstract
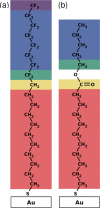
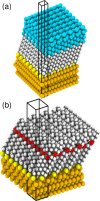
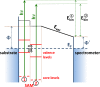
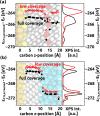
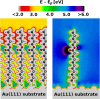
The focus of the present article is on understanding the insight that X-ray photoelectron spectroscopy (XPS) measurements can provide when studying self-assembled monolayers. Comparing density functional theory calculations to experimental data on deliberately chosen model systems, we show that both the chemical environment and electrostatic effects arising from a superposition of molecular dipoles influence the measured core-level binding energies to a significant degree. The crucial role of the often overlooked electrostatic effects in polar self-assembled monolayers (SAMs) is unambiguously demonstrated by changing the dipole density through varying the SAM coverage. As a consequence of this effect, care has to be taken when extracting chemical information from the XP spectra of ordered organic adsorbate layers. Our results, furthermore, imply that XPS is a powerful tool for probing local variations in the electrostatic energy in nanoscopic systems, especially in SAMs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Nuzzo R. G.; Allara D. L. Adsorption of Bifunctional Organic Disulfides on Gold Surfaces. J. Am. Chem. Soc. 1983, 105, 4481–4483. 10.1021/ja00351a063. - DOI
-
- Ulman A.An Introduction to Ultrathin Organic Films: From Langmuir-Blodgett to Self-Assembly; Academic Press: Boston, 1991.
-
- Feng X. J.; Jiang L. Design and Creation of Superwetting/Antiwetting Surfaces. Adv. Mater. 2006, 18, 3063–3078. 10.1002/adma.200501961. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
