Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: A Fabry Outcome Survey analysis
- PMID: 26937390
- PMCID: PMC4750577
- DOI: 10.1016/j.ymgmr.2015.02.002
Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: A Fabry Outcome Survey analysis
Abstract
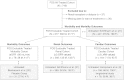
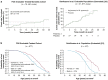
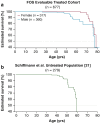
Outcomes from 5 years of treatment with agalsidase alfa enzyme replacement therapy (ERT) for Fabry disease in patients enrolled in the Fabry Outcome Survey (FOS) were compared with published findings for untreated patients with Fabry disease. Data were extracted from FOS, a Shire-sponsored database, for comparison with data from three published studies. Outcomes evaluated were the annualized rate of change in estimated glomerular filtration rate (eGFR) and left ventricular mass indexed to height (LVMI) as well as time to and ages at a composite morbidity endpoint and at death. FOS data were extracted for 740 treated patients who were followed for a median of ~ 5 years. Compared with no treatment, patients treated with agalsidase alfa demonstrated slower decline in renal function and slower progression of left ventricular hypertrophy. Treated male patients with baseline eGFR < 60 mL/min/1.73 m(2) had a mean (standard error of the mean [SEM]) annualized change in eGFR of - 2.86 (0.53) mL/min/1.73 m(2)/y compared with - 6.8 (1.5) in the published untreated cohort. The mean (SEM) rate of LVMI increase with treatment was 0.33 (0.10) g/m(2.7)/y in males and 0.48 (0.09) in females, compared with 4.07 (1.03) in untreated males and 2.31 (0.81) in untreated females. Morbidity occurred later in treated patients, with ~ 16% risk of a composite morbidity event (26% in males) after 24 months with ERT versus ~ 45% without treatment, with first events and deaths also occurring at older ages in patients administered ERT (e.g., estimated median survival in treated males was 77.5 years versus 60 years in untreated males). Findings from these retrospective comparisons of observational data and published literature support the long-term benefits of ERT with agalsidase alfa for Fabry disease in slowing the progression of renal impairment and cardiomyopathy. Treatment also appeared to delay the onset of morbidity and mortality. Interpretation of these findings should take into account that they are based on retrospective comparisons with previously published data.
Keywords: ACEI, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin receptor blocker; Agalsidase alfa; CI, Confidence interval; ERT, Enzyme replacement therapy; Enzyme replacement therapy; FOS, Fabry Outcome Survey; Fabry disease; LVH, Left ventricular hypertrophy; LVMI, Left ventricular mass indexed to height; Long-term effectiveness; MDRD, Modification of Diet in Renal Disease; SE, Standard error; SEM, Standard error of the mean; eGFR, Estimated glomerular filtration rate.
Figures
References
-
- Garman S.C., Garboczi D.N. The molecular defect leading to Fabry disease: structure of human alpha-galactosidase. J. Mol. Biol. 2004;337:319–335. - PubMed
-
- Mehta A., Clarke J.T., Giugliani R. Natural course of Fabry disease: changing pattern of causes of death in FOS — Fabry Outcome Survey. J. Med. Genet. 2009;46:548–552. - PubMed
-
- MacDermot K.D., Holmes A., Miners A.H. Natural history of Fabry disease in affected males and obligate carrier females. J. Inherit. Metab. Dis. 2001;24(Suppl. 2):13–14. (discussion 11–12) - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

