An Fe-N₂ Complex That Generates Hydrazine and Ammonia via Fe═NNH₂: Demonstrating a Hybrid Distal-to-Alternating Pathway for N₂ Reduction
- PMID: 26937584
- PMCID: PMC5065353
- DOI: 10.1021/jacs.6b01230
An Fe-N₂ Complex That Generates Hydrazine and Ammonia via Fe═NNH₂: Demonstrating a Hybrid Distal-to-Alternating Pathway for N₂ Reduction
Abstract
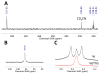
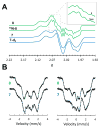
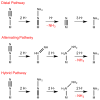
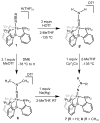
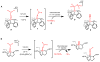
Biological N2 fixation to NH3 may proceed at one or more Fe sites in the active-site cofactors of nitrogenases. Modeling individual e(-)/H(+) transfer steps of iron-ligated N2 in well-defined synthetic systems is hence of much interest but remains a significant challenge. While iron complexes have been recently discovered that catalyze the formation of NH3 from N2, mechanistic details remain uncertain. Herein, we report the synthesis and isolation of a diamagnetic, 5-coordinate Fe═NNH2(+) species supported by a tris(phosphino)silyl ligand via the direct protonation of a terminally bound Fe-N2(-) complex. The Fe═NNH2(+) complex is redox-active, and low-temperature spectroscopic data and DFT calculations evidence an accumulation of significant radical character on the hydrazido ligand upon one-electron reduction to S = (1)/2 Fe═NNH2. At warmer temperatures, Fe═NNH2 rapidly converts to an iron hydrazine complex, Fe-NH2NH2(+), via the additional transfer of proton and electron equivalents in solution. Fe-NH2NH2(+) can liberate NH3, and the sequence of reactions described here hence demonstrates that an iron site can shuttle from a distal intermediate (Fe═NNH2(+)) to an alternating intermediate (Fe-NH2NH2(+)) en route to NH3 liberation from N2. It is interesting to consider the possibility that similar hybrid distal/alternating crossover mechanisms for N2 reduction may be operative in biological N2 fixation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
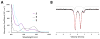
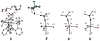
Similar articles
-
Vanadium-Catalyzed Dinitrogen Reduction to Ammonia via a [V]═NNH2 Intermediate.J Am Chem Soc. 2023 Jan 18;145(2):811-821. doi: 10.1021/jacs.2c08000. Epub 2023 Jan 3. J Am Chem Soc. 2023. PMID: 36596224
-
Catalytic reduction of hydrazine to ammonia by a mononuclear iron(II) complex on a tris(thiolato)phosphine platform.Inorg Chem. 2014 Jan 21;53(2):664-6. doi: 10.1021/ic402108w. Epub 2013 Dec 30. Inorg Chem. 2014. PMID: 24377381
-
N-H Bond Dissociation Enthalpies and Facile H Atom Transfers for Early Intermediates of Fe-N2 and Fe-CN Reductions.J Am Chem Soc. 2017 Mar 1;139(8):3161-3170. doi: 10.1021/jacs.6b12861. Epub 2017 Feb 17. J Am Chem Soc. 2017. PMID: 28140600 Free PMC article.
-
Catalytic N2-to-NH3 (or -N2H4) Conversion by Well-Defined Molecular Coordination Complexes.Chem Rev. 2020 Jun 24;120(12):5582-5636. doi: 10.1021/acs.chemrev.9b00638. Epub 2020 Apr 30. Chem Rev. 2020. PMID: 32352271 Free PMC article. Review.
-
Homogeneous iron complexes for the conversion of dinitrogen into ammonia and hydrazine.Chem Soc Rev. 2010 Nov;39(11):4044-56. doi: 10.1039/b919680n. Epub 2010 Jun 23. Chem Soc Rev. 2010. PMID: 20571678 Review.
Cited by
-
Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach.Sci Adv. 2018 Mar 9;4(3):eaar3208. doi: 10.1126/sciadv.aar3208. eCollection 2018 Mar. Sci Adv. 2018. PMID: 29536047 Free PMC article.
-
Identification of M-NH2 -NH2 Intermediate and Rate Determining Step for Nitrogen Reduction with Bioinspired Sulfur-Bonded FeW Catalyst.Angew Chem Int Ed Engl. 2021 Sep 6;60(37):20331-20341. doi: 10.1002/anie.202104918. Epub 2021 Aug 6. Angew Chem Int Ed Engl. 2021. PMID: 34245082 Free PMC article.
-
Exploring secondary-sphere interactions in Fe-N x H y complexes relevant to N2 fixation.Chem Sci. 2017 Mar 1;8(3):2321-2328. doi: 10.1039/c6sc04805f. Epub 2016 Dec 8. Chem Sci. 2017. PMID: 28451336 Free PMC article.
-
Rethinking the Nitrogenase Mechanism: Activating the Active Site.Joule. 2019 Nov 20;3(11):2662-2678. doi: 10.1016/j.joule.2019.09.004. Epub 2019 Oct 10. Joule. 2019. PMID: 32864580 Free PMC article.
-
The Spectroscopy of Nitrogenases.Chem Rev. 2020 Jun 24;120(12):5005-5081. doi: 10.1021/acs.chemrev.9b00650. Epub 2020 Apr 2. Chem Rev. 2020. PMID: 32237739 Free PMC article. Review.
References
-
- Howard JB, Rees DC. Chem Rev. 1996;96:2965–2982. - PubMed
-
- Lancaster KM, Roemelt M, Ettenhuber P, Hu Y, Ribbe MW, Neese F, Bergmann U, DeBeer S. Science. 2011;334:974–977. - PMC - PubMed
- Seefeldt LC, Hoffman BM, Dean DR. Annu Rev Biochem. 2009;78:701–722. - PMC - PubMed
- Spatzal T, Perez KA, Einsle O, Howard JB, Rees DC. Science. 2014;345:1620–1623. - PMC - PubMed
- Hinnemann B, Norskov JK. Top Catal. 2006;37:55–70.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

