Vaccines for preventing herpes zoster in older adults
- PMID: 26937872
- PMCID: PMC6516976
- DOI: 10.1002/14651858.CD008858.pub3
Vaccines for preventing herpes zoster in older adults
Update in
-
Vaccines for preventing herpes zoster in older adults.Cochrane Database Syst Rev. 2019 Nov 7;2019(11):CD008858. doi: 10.1002/14651858.CD008858.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Oct 2;10:CD008858. doi: 10.1002/14651858.CD008858.pub5. PMID: 31696946 Free PMC article. Updated.
Abstract
Background: Herpes zoster, also known as 'shingles', is a neurocutaneous disease characterised by the reactivation of the latent varicella zoster virus (VZV), the virus that causes chickenpox when immunity to VZV declines. It is an extremely painful condition that can last many weeks or months and it can significantly compromise the quality of life of affected individuals. The natural process of aging is associated with a reduction in cellular immunity and this predisposes older people to herpes zoster. Vaccination with an attenuated form of VZV activates specific T cell production avoiding viral reactivation. The Food and Drug Administration has approved a herpes zoster vaccine with an attenuated active virus for clinical use among older adults, which has been tested in large populations. A new adjuvanted recombinant VZV subunit zoster vaccine has also been tested. It consists of recombinant VZV glycoprotein E and a liposome-based AS01B adjuvant system. This new vaccine is not yet available for clinical use.
Objectives: To evaluate the effectiveness and safety of vaccination for preventing herpes zoster in older adults.
Search methods: For this 2015 update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 9), MEDLINE (1948 to the 3rd week of October 2015), EMBASE (2010 to October 2015), CINAHL (1981 to October 2015) and LILACS (1982 to October 2015).
Selection criteria: Randomised controlled trials (RCTs) or quasi-RCTs comparing zoster vaccine with placebo or no vaccine, to prevent herpes zoster in older adults (mean age > 60 years).
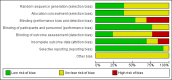
Data collection and analysis: Two review authors independently collected and analysed data using a data extraction form. They also performed 'Risk of bias' assessment.
Main results: We identified 13 studies involving 69,916 participants. The largest study included 38,546 participants. All studies were conducted in high-income countries and included only healthy Caucasian individuals ≥ 60 years of age without immunosuppressive comorbidities. Ten studies used live attenuated varicella zoster virus (VZV) vaccines. Three studies tested a new type of vaccine not yet available for clinical use. We judged five of the included studies to be at low risk of bias.The incidence of herpes zoster, at up to three years of follow-up, was lower in participants who received the vaccine than in those who received a placebo: risk ratio (RR) 0.49; 95% confidence interval (CI) 0.43 to 0.56, risk difference (RD) 2%, number needed to treat to benefit (NNTB) 50; GRADE: moderate quality evidence. The vaccinated group had a higher incidence of mild to moderate intensity adverse events. These date came from one large study that included 38,546 people aged 60 years or older.A study including 8122 participants compared the new vaccine (not yet available) to the placebo; the group that received the new vaccine had a lower incidence of herpes zoster at 3.2 years of follow-up: RR 0.04, 95% CI 0.02 to 0.10, RD 3%, NNTB 33; GRADE: moderate quality evidence. The vaccinated group had a higher incidence of adverse events but most them were of mild to moderate intensity.All studies received funding from the pharmaceutical industry.
Authors' conclusions: Herpes zoster vaccine is effective in preventing herpes zoster disease and this protection can last three years. In general, zoster vaccine is well tolerated; it produces few systemic adverse events and injection site adverse events of mild to moderate intensity.There are studies of a new vaccine (with a VZV glycoproteic fraction plus adjuvant), which is currently not yet available for clinical use.
Conflict of interest statement
Anna MZ Gagliardi: none known Brenda NG Andriolo: none known Maria R Torloni: none known Bernardo GO Soares: none known
Figures




































Update of
-
Vaccines for preventing herpes zoster in older adults.Cochrane Database Syst Rev. 2012 Oct 17;10:CD008858. doi: 10.1002/14651858.CD008858.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Mar 03;3:CD008858. doi: 10.1002/14651858.CD008858.pub3. PMID: 23076951 Updated.
References
References to studies included in this review
-
- Berger R, Trannoy E, Holländer G, Bailleux F, Rudin C, Creusvaux H. A dose‐response study of a live attenuated varicella‐zoster virus (Oka strain) vaccine administered to adults 55 years of age and older. Journal of Infectious Diseases 1998;178(Suppl 1):99‐103. [0022‐1899/98/78S1‐0022$02.00] - PubMed
- Trannoy E, Berger R, Holländer G, Bailleux F, Heimendinger P, Vuillier D, et al. Vaccination of immunocompetent elderly subjects with a live attenuated Oka strain of varicella zoster virus: a randomized, controlled, dose‐response trial. Vaccine 2000;18:1700‐6. [PII: S0264‐410X (99) 00510‐1] - PubMed
-
- Chlibek R, Bayas JM, Collins H, Pinta MLR, Ledent E, Johann F, et al. Safety and immunogenicity of an AS01 adjuvanted varicella‐zoster virus subunit candidate vaccine against herpes zoster in adults ≥ 50 years of age. Journal of Infectious Diseases 2013;208:1953–61. - PubMed
-
- Chlibek R, Smetana J, Pauksens K, Rombo L, Hoek JA, Richardus JH, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella‐zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014;32(15):1745‐53. - PubMed
-
- Diez‐Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, et al. Comparison of intramuscular and subcutaneous administration of a herpes zoster live‐attenuated vaccine in adults aged ≥50 years: a randomised non‐inferiority clinical trial. Vaccine 2015;33(6):789‐95. [MEDLINE: ] - PubMed
- Diez‐Domingo J, Weinke T, Kieninger‐Baum D, Eymin C, Thomas S, Sadorged C. A clinical study of a shingles (herpes zoster) vaccine (live) administered by intramuscular or subcutaneous routes in adults aged ≥ 50 years. European Geriatric Medicine 2013;4(Suppl):81–141.
-
- Gilderman LI, Lawless JF, Nolen TM, Sterling T, Rutledge RZ, Fernsler DA, et al. A double‐blind, randomized, controlled, multicenter safety and immunogenicity study of a refrigerator‐stable formulation of Zostavax. Clinical and Vaccine Immunology 2008;15(2):314‐9. [DOI: 10.1128/CVI.00310-07] - DOI - PMC - PubMed
References to studies excluded from this review
-
- Hayward AR, Buda K, Levin MJ. Immune response to secondary immunization with live or Inactivated VZV vaccine in elderly adults. Viral Immunology 1994;7(1):31‐6. [PUBMED: 7986334] - PubMed
-
- Hayward AR, Buda K, Jones M, White C Jo, Levin MJ. Varicella zoster virus specific cytotoxicity following secondary immunization with live or killed vaccine. Viral Immunology 1996;9(4):241‐5. [PUBMED: 8978020] - PubMed
-
- Kerzner B, Murray AV, Gheng E, Ifle R, Harvey PR, Tomlinson M, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. Journal of the American Geriatrics Society 2007;55(10):1499‐507. [DOI: 10.1111/j.1532-5415.2007.01397.x] - DOI - PubMed
-
- Leroux‐Roels I, Leroux‐Roels G, Frédéric Clement F, Vandepapelière P, Vassilev V, Ledent E, et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein E subunit vaccine candidate in young and older adults. Journal of Infectious Diseases 2012;206:1280–90. - PubMed
References to ongoing studies
-
- 'A double‐blind, randomised, placebo‐controlled, parallel group study to evaluate biomarkers of immunity to varicella zoster virus following immunisation with V212/heat‐treated varicella‐zoster virus (VZV) vaccine or with ZOSTAVAX in healthy volunteers'. Ongoing studyMarch 2009.
-
- 'Efficacy, safety, and immunogenicity study of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults aged ≥ 50 years'. Ongoing studyAugust 2010.
-
- 'Efficacy, safety and immunogenicity of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults aged >= 70 years'. Ongoing studyAugust 2010.
-
- 'A partially blinded randomised clinical trial to study the immunogenicity and safety of intradermal administration of ZOSTAVAX™ (V211)'. Ongoing studySeptember 2011.
-
- 'A phase III double‐blinded, randomised, multicenter, controlled study to evaluate the safety, tolerability, and immunogenicity of ZOSTAVAX™ made with an alternative manufacturing process (AMP)'. Ongoing studyApril 2012.
Additional references
-
- Arvin A. Ageing, immunity, and the varicella‐zoster virus. New England Journal of Medicine 2005;352(22):2266‐7. [PUBMED: 15930416] - PubMed
-
- Arvin AM, Kinney‐Thomas E, Shriver K, Grose C, Koropchak CM, Scranton E, et al. Immunity to varicella‐zoster viral glycoproteins, gp I (gp 90/58) and gp III (gp 118), and to a nonglycosylated protein, p 170. Journal of Immunology 1986;137(4):1346‐51. [PMID: 3016094] - PubMed
-
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, el al. Taking a toll on human disease: toll‐like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opinion on Biological Therapy 2004;4:1129‐38. [PMID: 15268679] - PubMed
References to other published versions of this review
-
- Gagliardi AMZ, Gomes SBN, Torloni MR, Soares BGO. Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008858] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

