Foxn1 Transcription Factor Regulates Wound Healing of Skin through Promoting Epithelial-Mesenchymal Transition
- PMID: 26938103
- PMCID: PMC4777299
- DOI: 10.1371/journal.pone.0150635
Foxn1 Transcription Factor Regulates Wound Healing of Skin through Promoting Epithelial-Mesenchymal Transition
Abstract
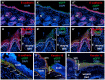
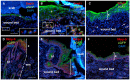
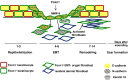
Transcription factors are key molecules that finely tune gene expression in response to injury. We focused on the role of a transcription factor, Foxn1, whose expression is limited to the skin and thymus epithelium. Our previous studies showed that Foxn1 inactivity in nude mice creates a pro-regenerative environment during skin wound healing. To explore the mechanistic role of Foxn1 in the skin wound healing process, we analyzed post-injured skin tissues from Foxn1::Egfp transgenic and C57BL/6 mice with Western Blotting, qRT-PCR, immunofluorescence and flow cytometric assays. Foxn1 expression in non-injured skin localized to the epidermis and hair follicles. Post-injured skin tissues showed an intense Foxn1-eGFP signal at the wound margin and in leading epithelial tongue, where it co-localized with keratin 16, a marker of activated keratinocytes. This data support the concept that suprabasal keratinocytes, expressing Foxn1, are key cells in the process of re-epithelialization. The occurrence of an epithelial-mesenchymal transition (EMT) was confirmed by high levels of Snail1 and Mmp-9 expression as well as through co-localization of vimentin/E-cadherin-positive cells in dermis tissue at four days post-wounding. Involvement of Foxn1 in the EMT process was verified by co-localization of Foxn1-eGFP cells with Snail1 in histological sections. Flow cytometric analysis showed the increase of double positive E-cadherin/N-cadherin cells within Foxn1-eGFP population of post-wounded skin cells isolates, which corroborated histological and gene expression analyses. Together, our findings indicate that Foxn1 acts as regulator of the skin wound healing process through engagement in re-epithelization and possible involvement in scar formation due to Foxn1 activity during the EMT process.
Conflict of interest statement
Figures






References
-
- Colwell AS, Longaker MT, Lorenz HP. Mammalian fetal organ regeneration. Adv Biochem Eng Biotechnol. 2005;93:83–100. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

