Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications
- PMID: 26938856
- PMCID: PMC5588529
- DOI: 10.1159/000445116
Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications
Abstract
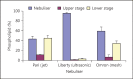
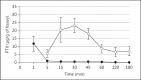
This is a critical review on research conducted in the field of pulmonary delivery of liposomes. Issues relating to the mechanism of nebulisation and liposome composition were appraised and correlated with literature reports of liposome formulations used in clinical trials to understand the role of liposome size and composition on therapeutic outcome. A major highlight was liposome inhalation for the treatment of lung cancers. Many in vivo studies that explored the potential of liposomes as anticancer carrier systems were evaluated, including animal studies and clinical trials. Liposomes can entrap anticancer drugs and localise their action in the lung following pulmonary delivery. The safety of inhaled liposomes incorporating anticancer drugs depends on the anticancer agent used and the amount of drug delivered to the target cancer in the lung. The difficulty of efficient targeting of liposomal anticancer aerosols to the cancerous tissues within the lung may result in low doses reaching the target site. Overall, following the success of liposomes as inhalable carriers in the treatment of lung infections, it is expected that more focus from research and development will be given to designing inhalable liposome carriers for the treatment of other lung diseases, including pulmonary cancers. The successful development of anticancer liposomes for inhalation may depend on the future development of effective aerosolisation devices and better targeted liposomes to maximise the benefit of therapy and reduce the potential for local and systemic adverse effects.
© 2016 S. Karger AG, Basel.
Figures
References
-
- American Cancer Society Cancer facts and figures 2013. Atlanta. http://www.cancer.org/acs/groups/content/@epidemiologysurveillance/docum... (accessed May 31, 2015).
-
- Sihoe AD, Yim AP. Lung cancer staging. J Surg Res. 2004;117:92–106. - PubMed
-
- Senan S, Paul MA, Lagerwaard FJ. Treatment of early-stage lung cancer detected by screening: surgery or stereotactic ablative radiotherapy? Lancet Oncol. 2013;14:270–274. - PubMed
-
- Hu L, Liang G, Yuliang W, et al. Assessing the effectiveness and safety of liposomal paclitaxel in combination with cisplatin as first-line chemotherapy for patients with advanced NSCLC with regional lymph-node metastasis: study protocol for a randomized controlled trial (PLC-GC trial) Trials. 2013;14:45. - PMC - PubMed
-
- Kadam AN, Najlah M, Wan KW, et al. Stability of parenteral nanoemulsions loaded with paclitaxel: the influence of lipid phase composition, drug concentration and storage temperature. Pharm Dev Technol. 2014;19:999–1004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

