Morphology and Molecular Composition of Purified Bovine Viral Diarrhea Virus Envelope
- PMID: 26939061
- PMCID: PMC4777508
- DOI: 10.1371/journal.ppat.1005476
Morphology and Molecular Composition of Purified Bovine Viral Diarrhea Virus Envelope
Abstract
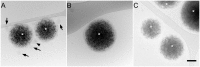
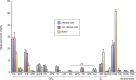
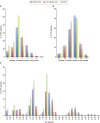
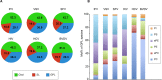
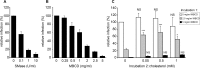
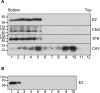
The family Flaviviridae includes viruses that have different virion structures and morphogenesis mechanisms. Most cellular and molecular studies have been so far performed with viruses of the Hepacivirus and Flavivirus genera. Here, we studied bovine viral diarrhea virus (BVDV), a member of the Pestivirus genus. We set up a method to purify BVDV virions and analyzed their morphology by electron microscopy and their protein and lipid composition by mass spectrometry. Cryo-electron microscopy showed near spherical viral particles displaying an electron-dense capsid surrounded by a phospholipid bilayer with no visible spikes. Most particles had a diameter of 50 nm and about 2% were larger with a diameter of up to 65 nm, suggesting some size flexibility during BVDV morphogenesis. Morphological and biochemical data suggested a low envelope glycoprotein content of BVDV particles, E1 and E2 being apparently less abundant than Erns. Lipid content of BVDV particles displayed a ~2.3 to 3.5-fold enrichment in cholesterol, sphingomyelin and hexosyl-ceramide, concomitant with a 1.5 to 5-fold reduction of all glycerophospholipid classes, as compared to lipid content of MDBK cells. Although BVDV buds in the endoplasmic reticulum, its lipid content differs from a typical endoplasmic reticulum membrane composition. This suggests that BVDV morphogenesis includes a mechanism of lipid sorting. Functional analyses confirmed the importance of cholesterol and sphingomyelin for BVDV entry. Surprisingly, despite a high cholesterol and sphingolipid content of BVDV envelope, E2 was not found in detergent-resistant membranes. Our results indicate that there are differences between the structure and molecular composition of viral particles of Flaviviruses, Pestiviruses and Hepaciviruses within the Flaviviridae family.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Characterization and purification of recombinant bovine viral diarrhea virus particles with epitope-tagged envelope proteins.J Gen Virol. 2011 Jun;92(Pt 6):1352-1357. doi: 10.1099/vir.0.029330-0. Epub 2011 Feb 23. J Gen Virol. 2011. PMID: 21346033
-
Morphology of bovine viral diarrhea virus.Am J Vet Res. 1984 May;45(5):845-50. Am J Vet Res. 1984. PMID: 6329046
-
Inhibition of host ER glucosidase activity prevents Golgi processing of virion-associated bovine viral diarrhea virus E2 glycoproteins and reduces infectivity of secreted virions.Virology. 2002 Mar 30;295(1):10-9. doi: 10.1006/viro.2002.1370. Virology. 2002. PMID: 12033761
-
Electron microscopy of bovine virus diarrhoea virus.Rev Sci Tech. 1990 Mar;9(1):61-73. doi: 10.20506/rst.9.1.490. Rev Sci Tech. 1990. PMID: 1966727 Review.
-
[Bovine diarrhea virus: an update].Rev Argent Microbiol. 1997 Jan-Mar;29(1):47-61. Rev Argent Microbiol. 1997. PMID: 9229725 Review. Spanish.
Cited by
-
Cell-to-Cell Transmission Is the Main Mechanism Supporting Bovine Viral Diarrhea Virus Spread in Cell Culture.J Virol. 2019 Jan 17;93(3):e01776-18. doi: 10.1128/JVI.01776-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30404802 Free PMC article.
-
Broad-spectrum antiviral agents: secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane.Sci Rep. 2017 Nov 21;7(1):15931. doi: 10.1038/s41598-017-16130-w. Sci Rep. 2017. PMID: 29162867 Free PMC article.
-
Stearoly-CoA desaturase 1 differentiates early and advanced dengue virus infections and determines virus particle infectivity.PLoS Pathog. 2018 Aug 17;14(8):e1007261. doi: 10.1371/journal.ppat.1007261. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30118512 Free PMC article.
-
Maternal Magnolol Supplementation during Pregnancy and Lactation Promotes Antioxidant Capacity, Improves Gut Health, and Alters Gut Microbiota and Metabolites of Weanling Piglets.Metabolites. 2023 Jun 27;13(7):797. doi: 10.3390/metabo13070797. Metabolites. 2023. PMID: 37512505 Free PMC article.
-
Congenital infection with atypical porcine pestivirus (APPV) is associated with disease and viral persistence.Vet Res. 2017 Jan 6;48(1):1. doi: 10.1186/s13567-016-0406-1. Vet Res. 2017. PMID: 28057061 Free PMC article.
References
-
- Lindenbach BD, Thiel H-J, Rice CM. Flaviviridae: the viruses and their replication. Knipe DM, Howley PM, editors. Fields Virology. 5 ed. Philadelphia; 2007;: 1101–1152.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

