Case-fatality risk of pregnant women with acute viral hepatitis type E: a systematic review and meta-analysis
- PMID: 26939626
- PMCID: PMC9150575
- DOI: 10.1017/S0950268816000418
Case-fatality risk of pregnant women with acute viral hepatitis type E: a systematic review and meta-analysis
Abstract
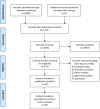
It is of great concern that pregnant women with acute viral hepatitis (AVH) type E have serious consequences. This study aimed to estimate the case-fatality risk (CFR) and potential risk factors of pregnant women with AVH type E. We searched the PubMed, EMBASE, and Web of Science databases for studies containing data on CFR in pregnancy with AVH type E. A pooled estimate of CFR was calculated using a random-effects model. Potential sources of heterogeneity were explored using subgroup analysis, sensitivity analysis, and meta-regression. We identified 47 eligible studies with a total African and Asian population of 3968 individuals. The pooled CFRs of maternal and fetal outcomes were 20·8% [95% confidence interval (CI) 16·6-25·3] and 34·2% (95% CI 26·0-43·0), respectively. Compared with these, the pooled CFR was highest (61·2%) in women with fulminant hepatic failure (FHF). Community-based surveys had lower pooled CFR (12·2%, 95% CI 9·2-15·6) and heterogeneity (25·8%, 95% CI 20·1-32·0) than hospital-based surveys. Univariate analysis showed that hospital-based surveying (P = 0·007), and patients in the third trimester of pregnancy or with FHF (P < 0·05), were significantly associated with CFR. Intrauterine fetal mortality (27·0%) was statistically higher than neonatal mortality (3·9%). Control measures for HEV infection would reduce feto-maternal mortality in Asia and Africa.
Keywords: Acute viral hepatitis E; case-fatality risk; meta-analysis; pregnancy.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

