The need for second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030: a mathematical modelling study
- PMID: 26939736
- PMCID: PMC5688234
- DOI: 10.1016/S2352-3018(16)00016-3
The need for second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030: a mathematical modelling study
Abstract
Background: The number of patients in need of second-line antiretroviral drugs is increasing in sub-Saharan Africa. We aimed to project the need of second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030.
Methods: We developed a simulation model for HIV and applied it to each sub-Saharan African country. We used the WHO country intelligence database to estimate the number of adult patients receiving antiretroviral therapy from 2005 to 2014. We fitted the number of adult patients receiving antiretroviral therapy to observed estimates, and predicted first-line and second-line needs between 2015 and 2030. We present results for sub-Saharan Africa, and eight selected countries. We present 18 scenarios, combining the availability of viral load monitoring, speed of antiretroviral scale-up, and rates of retention and switching to second-line. HIV transmission was not included.
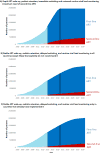
Findings: Depending on the scenario, 8·7-25·6 million people are expected to receive antiretroviral therapy in 2020, of whom 0·5-3·0 million will be receiving second-line antiretroviral therapy. The proportion of patients on treatment receiving second-line therapy was highest (15·6%) in the scenario with perfect retention and immediate switching, no further scale-up, and universal routine viral load monitoring. In 2030, the estimated range of patients receiving antiretroviral therapy will remain constant, but the number of patients receiving second-line antiretroviral therapy will increase to 0·8-4·6 million (6·6-19·6%). The need for second-line antiretroviral therapy was two to three times higher if routine viral load monitoring was implemented throughout the region, compared with a scenario of no further viral load monitoring scale-up. For each monitoring strategy, the future proportion of patients receiving second-line antiretroviral therapy differed only minimally between countries.
Interpretation: Donors and countries in sub-Saharan Africa should prepare for a substantial increase in the need for second-line drugs during the next few years as access to viral load monitoring improves. An urgent need exists to decrease the costs of second-line drugs.
Funding: World Health Organization, Swiss National Science Foundation, National Institutes of Health.
Copyright © 2016 World Health Organization. Published by Elsevier Ltd/Inc/BV. All rights reserved. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
Comment in
-
First-line HIV therapy shall not fail.Lancet HIV. 2016 Mar;3(3):e108-9. doi: 10.1016/S2352-3018(16)00022-9. Epub 2016 Feb 16. Lancet HIV. 2016. PMID: 26939732 No abstract available.
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) AIDSInfo Online Database. Geneva, Switzerland: UNAIDS; p. 2015. Available from: www.aidsinfoonline.org. Accessed 27 December 2015.
-
- World Health Organization. Recommendations for a public health approach. Geneva, Switzerland: World Health Organization; Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; p. 2013. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/en/. Accessed 6 November 2015. - PubMed
-
- Médecins Sans Frontières. Untangling the Web of Antiretroviral Price Reductions. 17th. Geneva, Switzerland: Médecins Sans Frontières; 2014. Available from: http://www.msfaccess.org/content/untangling-web-antiretroviral-price-red.... Accessed 6 November 2015.
-
- Rutherford GW, Anglemyer A, Easterbrook PJ, et al. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS. 2014;28(Suppl 2):S161–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

