Stereological analyses of reward system nuclei in maternally deprived/separated alcohol drinking rats
- PMID: 26939765
- PMCID: PMC5010523
- DOI: 10.1016/j.jchemneu.2016.02.004
Stereological analyses of reward system nuclei in maternally deprived/separated alcohol drinking rats
Abstract
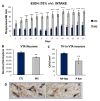
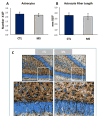
The experience of early life stress can trigger complex neurochemical cascades that influence emotional and addictive behaviors later in life in both adolescents and adults. Recent evidence suggests that excessive alcohol drinking and drug-seeking behavior, in general, are co-morbid with depressive-like behavior. Both behaviors are reported in humans exposed to early life adversity, and are prominent features recapitulated in animal models of early life stress (ELS) exposure. Currently, little is known about whether or how ELS modulates reward system nuclei. In this study we use operant conditioning of rats to show that the maternal separation stress (MS) model of ELS consumes up to 3-fold greater quantities of 10% vol/vol EtOH in 1-h, consistently over a 3-week period. This was correlated with a significant 22% reduction in the number of dopaminergic-like neurons in the VTA of naïve MS rats, similar to genetically alcohol-preferring (P) rats which show a 35% reduction in tyrosine hydroxylase (TH)-positive dopaminergic neurons in the VTA. MS rats had a significantly higher 2-fold immobility time in the forced swim test (FST) and reduced sucrose drinking compared to controls, indicative of depressive-like symptomology and anhedonia. Consistent with this finding, stereological analysis revealed that amygdala neurons were 25% greater in number at P70 following MS exposure. Our previous examination of the dentate gyrus of hippocampus, a region involved in encoding emotional memory, revealed fewer dentate gyrus neurons after MS, but we now report this reduction in neurons occurs without effect on the number of astrocytes or length of astrocytic fibers. These data indicate that MS animals exhibit neuroanatomical changes in reward centers similar to those reported for high alcohol drinking rats, but aspects of astrocyte morphometry remained unchanged. These data are of high relevance to understand the breadth of neuronal pathology that ensues in reward loci following ELS.
Keywords: Alcohol-preferring rats; Amygdala; Dentate gyrus; Drug addiction; Early life stress; Excessive alcohol drinking; Maternal deprivation; Maternal separation; P rats; Stereology; VTA.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Combined early life stressors: Prenatal nicotine and maternal deprivation interact to influence affective and drug seeking behavioral phenotypes in rats.Behav Brain Res. 2019 Feb 1;359:814-822. doi: 10.1016/j.bbr.2018.07.022. Epub 2018 Jul 25. Behav Brain Res. 2019. PMID: 30055209 Free PMC article.
-
Early-life stress increases the survival of midbrain neurons during postnatal development and enhances reward-related and anxiolytic-like behaviors in a sex-dependent fashion.Int J Dev Neurosci. 2015 Aug;44:33-47. doi: 10.1016/j.ijdevneu.2015.05.002. Epub 2015 May 14. Int J Dev Neurosci. 2015. PMID: 25980793
-
Locus coeruleus neuronal activity determines proclivity to consume alcohol in a selectively-bred line of rats that readily consumes alcohol.Alcohol. 2015 Nov;49(7):691-705. doi: 10.1016/j.alcohol.2015.08.008. Epub 2015 Sep 25. Alcohol. 2015. PMID: 26496795 Free PMC article.
-
[Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French.
-
Early life stress and susceptibility to addiction in adolescence.Int Rev Neurobiol. 2022;161:277-302. doi: 10.1016/bs.irn.2021.08.007. Epub 2021 Oct 9. Int Rev Neurobiol. 2022. PMID: 34801172 Review.
Cited by
-
Administration of a putative pro-dopamine regulator, a neuronutrient, mitigates alcohol intake in alcohol-preferring rats.Behav Brain Res. 2020 May 15;385:112563. doi: 10.1016/j.bbr.2020.112563. Epub 2020 Feb 15. Behav Brain Res. 2020. PMID: 32070691 Free PMC article.
-
Total Number Is Important: Using the Disector Method in Design-Based Stereology to Understand the Structure of the Rodent Brain.Front Neuroanat. 2018 Mar 5;12:16. doi: 10.3389/fnana.2018.00016. eCollection 2018. Front Neuroanat. 2018. PMID: 29556178 Free PMC article. Review.
-
Early life stress-induced alterations in the activity and morphology of ventral tegmental area neurons in female rats.Neurobiol Stress. 2020 Sep 3;13:100250. doi: 10.1016/j.ynstr.2020.100250. eCollection 2020 Nov. Neurobiol Stress. 2020. PMID: 33344705 Free PMC article.
-
Basic quantitative morphological methods applied to the central nervous system.J Comp Neurol. 2021 Mar;529(4):694-756. doi: 10.1002/cne.24976. Epub 2020 Aug 1. J Comp Neurol. 2021. PMID: 32639600 Free PMC article. Review.
-
The involvement of astrocytes in early-life adversity induced programming of the brain.Glia. 2019 Sep;67(9):1637-1653. doi: 10.1002/glia.23625. Epub 2019 Apr 30. Glia. 2019. PMID: 31038797 Free PMC article. Review.
References
-
- Abe K. Modulation of hippocampal long-term potentiation by the amygdala: a synaptic mechanism linking emotion and memory. Jpn J Pharmacol. 2001;86:18–22. - PubMed
-
- Abe K, Niikura Y, Fujimoto T, Akaishi T, Misawa M. Involvement of dopamine D2 receptors in the induction of long-term potentiation in the basolateral amygdala-dentate gyrus pathway of anesthetized rats. Neuropharmacology. 2008;55:1419–1424. - PubMed
-
- Aisa B, Elizalde N, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: implications for spatial memory. Hippocampus. 2009;19:1222–1231. - PubMed
-
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

