Glucosensing in the gastrointestinal tract: Impact on glucose metabolism
- PMID: 26939867
- PMCID: PMC4867329
- DOI: 10.1152/ajpgi.00015.2016
Glucosensing in the gastrointestinal tract: Impact on glucose metabolism
Abstract
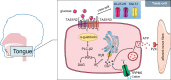
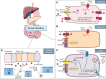
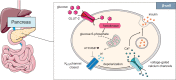
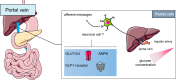
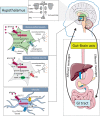
The gastrointestinal tract is an important interface of exchange between ingested food and the body. Glucose is one of the major dietary sources of energy. All along the gastrointestinal tube, e.g., the oral cavity, small intestine, pancreas, and portal vein, specialized cells referred to as glucosensors detect variations in glucose levels. In response to this glucose detection, these cells send hormonal and neuronal messages to tissues involved in glucose metabolism to regulate glycemia. The gastrointestinal tract continuously communicates with the brain, especially with the hypothalamus, via the gut-brain axis. It is now well established that the cross talk between the gut and the brain is of crucial importance in the control of glucose homeostasis. In addition to receiving glucosensing information from the gut, the hypothalamus may also directly sense glucose. Indeed, the hypothalamus contains glucose-sensitive cells that regulate glucose homeostasis by sending signals to peripheral tissues via the autonomous nervous system. This review summarizes the mechanisms by which glucosensors along the gastrointestinal tract detect glucose, as well as the results of such detection in the whole body, including the hypothalamus. We also highlight how disturbances in the glucosensing process may lead to metabolic disorders such as type 2 diabetes. A better understanding of the pathways regulating glucose homeostasis will further facilitate the development of novel therapeutic strategies for the treatment of metabolic diseases.
Keywords: diabetes; glucose homeostasis; glucosensing.
Copyright © 2016 the American Physiological Society.
Figures
References
-
- Adachi A, Shimizu N, Oomura Y, Kobashi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett 46: 215–218, 1984. - PubMed
-
- Ahren B. Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia 43: 393–410, 2000. - PubMed
-
- Ahren B. Glucagon–early breakthroughs and recent discoveries. Peptides 67: 74–81, 2015. - PubMed
-
- Amato A, Cinci L, Rotondo A, Serio R, Faussone-Pellegrini MS, Vannucchi MG, Mule F. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil 22: 664–e203, 2010. - PubMed
-
- Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol 279: R1449–R1454, 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

