Urine of Preterm Neonates as a Novel Source of Kidney Progenitor Cells
- PMID: 26940093
- PMCID: PMC5004650
- DOI: 10.1681/ASN.2015060664
Urine of Preterm Neonates as a Novel Source of Kidney Progenitor Cells
Abstract
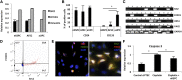
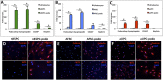
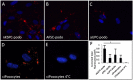
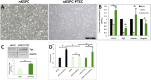
In humans, nephrogenesis is completed prenatally, with nephrons formed until 34 weeks of gestational age. We hypothesized that urine of preterm neonates born before the completion of nephrogenesis is a noninvasive source of highly potent stem/progenitor cells. To test this hypothesis, we collected freshly voided urine at day 1 after birth from neonates born at 31-36 weeks of gestational age and characterized isolated cells using a single-cell RT-PCR strategy for gene expression analysis and flow cytometry and immunofluorescence for protein expression analysis. Neonatal stem/progenitor cells expressed markers of nephron progenitors but also, stromal progenitors, with many single cells coexpressing these markers. Furthermore, these cells presented mesenchymal stem cell features and protected cocultured tubule cells from cisplatin-induced apoptosis. Podocytes differentiated from the neonatal stem/progenitor cells showed upregulation of podocyte-specific genes and proteins, albumin endocytosis, and calcium influx via podocyte-specific transient receptor potential cation channel, subfamily C, member 6. Differentiated proximal tubule cells showed upregulation of specific genes and significantly elevated p-glycoprotein activity. We conclude that urine of preterm neonates is a novel noninvasive source of kidney progenitors that are capable of differentiation into mature kidney cells and have high potential for regenerative kidney repair.
Keywords: preterm; stem cell; urine.
Copyright © 2016 by the American Society of Nephrology.
Figures


References
-
- Bard JB, McConnell JE, Davies JA: Towards a genetic basis for kidney development. Mech Dev 48: 3–11, 1994 - PubMed
-
- Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M: Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313: 234–245, 2008 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

